155877-83-1
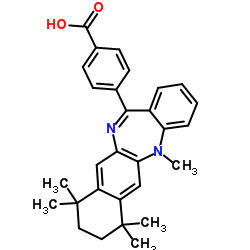
| Name | 4-(5,7,7,10,10-pentamethyl-8,9-dihydronaphtho[2,3-b][1,4]benzodiazepin-13-yl)benzoic acid |
|---|---|
| Synonyms |
4-(5,7,7,10,10-Pentamethyl-7,8,9,10-tetrahydro-5H-benzo[e]naphtho[2,3-b][1,4]diazepin-13-yl)benzoic acid
hms3268h07 le 135 |
| Description | LE135 is a potent RAR antagonist that binds selectively to RARα (Ki of 1.4 μM) and RARβ (Ki of 220 nM), and has a higher affinity to RARβ. LE135 is highly selective over RARγ, RXRα, RXRβ and RXRγ. LE135 is also a potent TRPV1 and TRPA1 receptors activator with EC50s of 2.5 μM and 20 μM, respectively[1][2]. |
|---|---|
| Related Catalog | |
| Target |
RARβ:220 nM (Ki) RARα:1400 nM (Ki) TRPV1:2.5 μM (EC50) TRPA1:20 μM (EC50) |
| In Vitro | LE135 inhibits Am80-induced differentiation of human promyelocytic leukemia cells HL-60 with an IC50 of 150 nM[1]. LE135 inhibits retinoic acid (RA)-induced transcriptional activation of RARβ, but not RARα, RARγ or retinoid X receptor α (RXRα), on a variety of RA response elements. LE135 strongly represses 12-O-tetradecanoylphorbol-13-acetate-induced AP-1 activity in the presence of RARβ and RXRα[3]. |
| In Vivo | LE135 provokes nociceptive responses and elicited thermal hyperalgesia mainly through TRPV1 channels, but required both TRPA1 and TRPV1 channels for producing mechanical allodynia. Intraplantar injection of LE135 (30 nmol/10 μL) induces mechanical hypersensitivity in wild-type and Trpa1−/− mice[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 601.3±55.0 °C at 760 mmHg |
| Molecular Formula | C29H30N2O2 |
| Molecular Weight | 438.561 |
| Flash Point | 317.5±31.5 °C |
| Exact Mass | 438.230713 |
| PSA | 52.90000 |
| LogP | 7.36 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.628 |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |