549-49-5
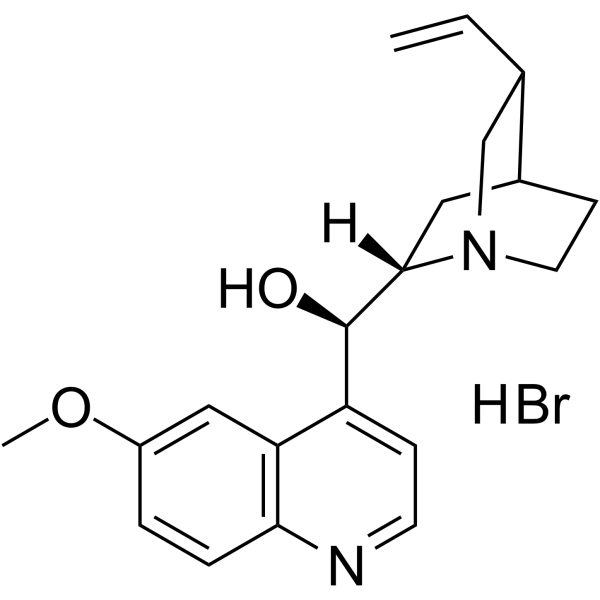
| Name | (R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl]-(6-methoxyquinolin-4-yl)methanol,hydrobromide |
|---|---|
| Synonyms |
Chinin hydrobromid
QUININE HYDROBROMIDE Quinine,monohydrobromide UNII-VWF36Q4G6V Chinin hydrobromid [German] Quinine hydrobromide [NF] EINECS 208-967-2 Bromoquinine |
| Description | Quinidine hydrobromide is an antiarrhythmic agent. Quinidine is a potent, orally active, selective cytochrome P450db inhibitor. Quinidine hydrobromide is also a K+ channel blocker with an IC50 of 19.9 μM. Quinidine hydrobromide can be used for malaria research[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Quinidine hydrobromide is an anti-arrythmic drug which affects ionic currents in heart muscle and which has also been shown to be a potent blocker of several classes of K+ channel in a variety of cell types[1]. Bath application of quinidine hydrobromide causes a dose-dependent reduction of the peak amplitude of Ik. The Kd for blockade of Ik at 0 mV is estimated to be 41 μM[1]. Quinidine hydrobromide elicits a dose-dependent increase of the rate of the decay of Ik and this effect is enhanced by membrane depolarization. Quinidine also causes a 5 mV hyperpolarizing shift of the steady-state inactivation curve and increases the half-time for recovery from inactivation. Quinidine hydrobromide does not affect the onset of inactivation measured at -30 mV[1]. |
| In Vivo | Quinidine hydrobromide is rapidly absorbed, with peak plasma concentrations 60-90 min after an oral dose. Other salts (gluconate, polygalacturonate) are more slowly absorbed, with lower peak concentrations[2]. Quinidine hydrobromide is approximately 70-90 % bound to plasma proteins. It undergoes hepatic oxidative metabolism to form an N-oxide, a 3-hydroxy form, an O-demethyl form and 2'-quinidinone[2]. Quinidine hydrobromide inhibits metabolism of amphetamine in rats. Quinidine hydrobromide pretreatment results in a significant decrease in the excretion of p-hydroxyamphetamine at 24 and 48 h to 7.2 and 24.1% of the vehicle-control levels, respectively, accompanied by a significant increase in amphetamine excretion between 24 and 48 h to 542% of the control[3]. |
| References |
| Density | 1.21g/cm3 |
|---|---|
| Boiling Point | 495.9ºC at 760mmHg |
| Melting Point | 81-82ºC |
| Molecular Formula | C20H25BrN2O2 |
| Molecular Weight | 405.32900 |
| Flash Point | 253.7ºC |
| Exact Mass | 404.11000 |
| PSA | 45.59000 |
| LogP | 4.06920 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn,Xi |
|---|---|
| Risk Phrases | 20/21/22-36/37/38-42/43 |
| RIDADR | UN 1544 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 29392000 |
| HS Code | 29392000 |
|---|