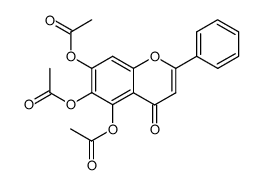
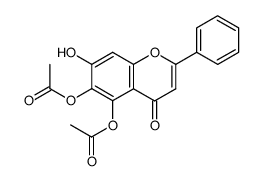
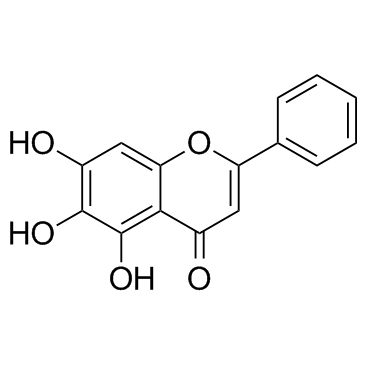
57396-78-8
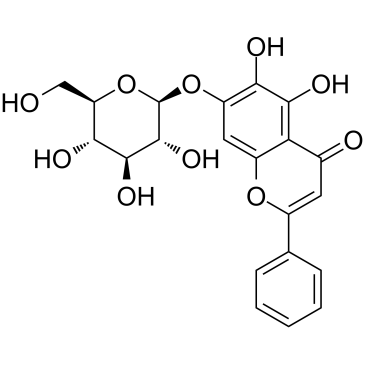
| Name | 5,6-Dihydroxy-4-oxo-2-phenyl-4H-chromen-7-yl β-D-glucopyranoside |
|---|---|
| Synonyms |
5,6-dihydroxyflavone-7-glucoside
B-Aethyl-boracycloheptan 5,6-Dihydroxy-4-oxo-2-phenyl-4H-chromen-7-yl β-D-glucopyranoside 1-ethyl-borepane baicalein-7-O-glucoside Borepane,1-ethyl Baicalein 7-b-D-glucopyranoside Oroxin A |
| Description | Oroxin A is the major component of an ethanol-water Oroxylum indicum (L.) Kurz (Bignoniaceae) seed extract (OISE), activates peroxisome proliferator-activated receptor γ (PPARγ) by docking into the PPARγ protein ligand-binding domain. Oroxin A exhibits an inhibitory activity against α-glucosidase and an antioxidant capacity[1]. Oroxin A exerts anti-breast cancer effects by inducing ER stress-mediated senescence[2]. |
|---|---|
| Related Catalog | |
| In Vitro | Oroxin A (0.5- 100 μM; 24 hours) significantly increases the PPARγ transcription level and exhibits the strongest activation with 50 μM in HEK-293t cells[1]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 784.0±60.0 °C at 760 mmHg |
| Molecular Formula | C21H20O10 |
| Molecular Weight | 432.378 |
| Flash Point | 279.0±26.4 °C |
| Exact Mass | 432.105652 |
| PSA | 170.05000 |
| LogP | 0.47 |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.717 |
| Storage condition | -20℃ |
| Hazard Codes | N |
|---|
|
~% 
57396-78-8 |
| Literature: Mezey-Vandor, Gabriella; Farkas, Lorand; Kanzel, Ida; Nogradi, Mihaly Chemische Berichte, 1980 , vol. 113, # 5 p. 1945 - 1949 |
|
~% 
57396-78-8 |
| Literature: Mezey-Vandor, Gabriella; Farkas, Lorand; Kanzel, Ida; Nogradi, Mihaly Chemische Berichte, 1980 , vol. 113, # 5 p. 1945 - 1949 |
|
~% 
57396-78-8 |
| Literature: Mezey-Vandor, Gabriella; Farkas, Lorand; Kanzel, Ida; Nogradi, Mihaly Chemische Berichte, 1980 , vol. 113, # 5 p. 1945 - 1949 |
|
~% 
57396-78-8 |
| Literature: Mezey-Vandor, Gabriella; Farkas, Lorand; Kanzel, Ida; Nogradi, Mihaly Chemische Berichte, 1980 , vol. 113, # 5 p. 1945 - 1949 |
|
~% 
57396-78-8 |
| Literature: Mezey-Vandor, Gabriella; Farkas, Lorand; Kanzel, Ida; Nogradi, Mihaly Chemische Berichte, 1980 , vol. 113, # 5 p. 1945 - 1949 |
| Precursor 5 | |
|---|---|
| DownStream 1 | |