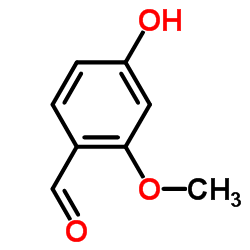
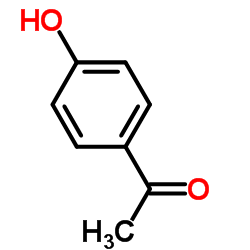
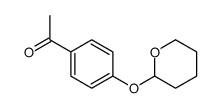
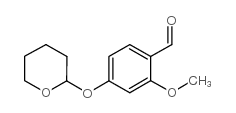
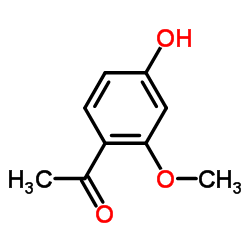
34221-41-5
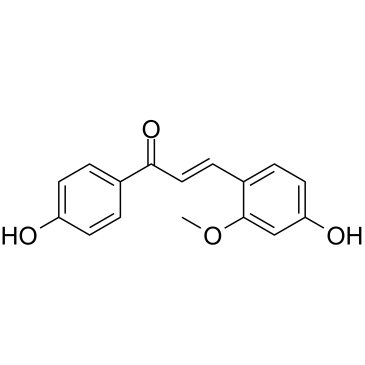
| Name | (E)-3-(4-hydroxy-2-methoxyphenyl)-1-(4-hydroxyphenyl)prop-2-en-1-one |
|---|---|
| Synonyms |
Echinatin
Retrochalcone (2E)-3-(4-Hydroxy-2-methoxyphenyl)-1-(4-hydroxyphenyl)-2-propen-1-one Echinantin 2-methoxy-4,4'-dihydroxychalcone (2E)-3-(4-hydroxy-2-methoxyphenyl)-1-(4-hydroxyphenyl)prop-2-en-1-one loureirin C (E)-4,4'-Dihydroxy-2-methoxychalcone 4',4-dihydroxy-2-methoxychalcone |
| Description | Echinatin is a chalcone isolated from the Chinese herbal medicine Gancao with hepatoprotective and anti-inflammatory effects. Echinatin may undergo an electron transfer (ET) and a proton transfer (PT) to cause the antioxidant action in aqueous solution[1]. Echinatin can be quickly absorbed and eliminated and extensively distributed with an absolute bioavailability of approximately 6.81% in Rat[2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 509.8±50.0 °C at 760 mmHg |
| Melting Point | 210ºC (dec.) |
| Molecular Formula | C16H14O4 |
| Molecular Weight | 270.280 |
| Flash Point | 193.3±23.6 °C |
| Exact Mass | 270.089203 |
| PSA | 66.76000 |
| LogP | 3.23 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.658 |
| Storage condition | 2-8°C |
| HS Code | 2914509090 |
|---|
|
~% 
34221-41-5 |
| Literature: Achenbach, Hans; Stoecker, Markus; Constenla, Manuel A. Phytochemistry (Elsevier), 1988 , vol. 27, # 6 p. 1835 - 1842 |
|
~% 
34221-41-5 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 23, # 11 p. 3320 - 3324 |
|
~% 
34221-41-5 |
| Literature: Phytochemistry (Elsevier), , vol. 19, p. 2179 - 2184 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |