50-65-7
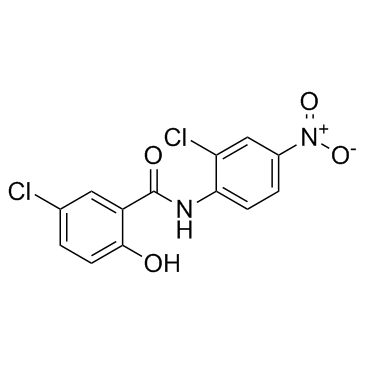
| Name | niclosamide |
|---|---|
| Synonyms |
2',5-Dichloro-4'-nitrosalicylanilide
2’,5-dichloro-4’-nitrosalicylanilide iomesan nasemo NICLOCIDE hl2447 Niclosamide clonitralid EINECS 200-056-8 iomezan bay2353 lintex MFCD00057597 sulqui 5-Chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxybenzamide mato fenasal 5-Chlor-N-(2-chlor-4-nitrophenyl)-2-hydroxybenzolcarboxamid b2353 |
| Description | Niclosamide is an inhibitor of STAT3 with IC50 of 0.25 μM in HeLa cells and inhibits DNA replication in a cell-free assay. |
|---|---|
| Related Catalog | |
| Target |
STAT3:0.25 μM (IC50, in HeLa cells) |
| In Vitro | Niclosamide is an inhibitor of STAT3, inhibiting STAT3-mediated luciferase reporter activity with an IC50 of 0.25 μM in HeLa cells. Niclosamide (1 μM) inhibits the EGF-induced nuclear translocation of STAT3 in Du145 cells. Niclosamide (< 2 μM) dose dependently inhibits the transcription of STAT3 downstream genes in Du145 cells. Niclosamide (< 10 μM) induces G0/G1 arrest and apoptosis of Du145 cancer cells in a dose dependent manner[1]. Niclosamide can block SARS-CoV replication at a micromolar concentration in Vero E6 cells infected with SARS-CoV[2]. Niclosamide (< 7.5 μM) promotes Frizzled1 endocytosis, downregulates Dishevelled-2 protein, and inhibits Wnt3A-stimulated beta-catenin stabilization and LEF/TCF reporter activity in U2OS cells[3]. Niclosamide inhibits the TNF-induced NF-κB reporter activity in a dose- and time-dependent manner in U2OS cells. Niclosamide (125 nM) inhibits NF-κB activation induced by p65, IKKα, IKKβ, IKKγ, and TAK1 in U2OS cells. Niclosamide (< 500 nM) completely block the time- and dose-dependent TNFα-induced alteration of the NF-κB-DNA complex in HL-60 cells. Niclosamide (< 10 nM) inhibits constitutive NF-κB activation in U266 cells. Niclosamide inhibits TNF-induced degradation of IκBα and relocation of p65 in a dose- and time-dependent manner in HL-60, Molm13, or AML primary cells. Niclosamide (500 nM) causes decrease in TNF-induced NF-κB-dependent gene products involved in cell survival in HL-60 cells. Niclosamide also inhibits the growth and induces robust apoptosis of AML cells associated with decreased Mcl-1 and XIAP levels and increased intracellular ROS levels[4]. |
| In Vivo | Niclosamide (40 mg/kg/d, i.p.) suppresses the growth of xenografted AML cells in nude mice bearing HL-60 xenograft tumors[4]. |
| Kinase Assay | All of the protein kinases are expressed either in Sf9 insect cells or in E.coli as recombinant GST-fusion proteins or His-tagged proteins. A radiometric protein kinase assay is used for measuring the kinase activity of the 22 protein kinases. Briefly, for each protein kinase, 50 μL reaction cocktail containing 60 mM HEPES-NaOH, 3 mM MgCl2, 3 mM MnCl2, 3 μM Na-orthovanadate, 1.2 mM DTT, 50 μg/mL PEG20000, 1 μM [γ-33P]-ATP, Niclosamide, adequate amount of enzyme and its substrate. The PKC-alpha assay additionally contain 1 mM Ca2, 4 mM EDTA, 5 μg/mL phosphatidylserine and 1 μg/mL 1, 2-Dioleyl-glycerol. The reaction cocktails are incubated at 37°C for 60 minutes and stop with 50 μL 2% (v/v) H3PO4. Incorporation of 33Pi is determined with a microplate scintillation counter. The activities and the IC50 values are calculated using Quattro Workflow V2.28. |
| Cell Assay | Cells are plated in 96-well culture plates with cell density of 3-4 ×103 cells/well and treat with Niclosamide by adding 100 μL medium containing Niclosamide of various concentrations on the second day. After 72-hour's treatment, MTT is added to each well and incubated for additional 4-5 hours, and the absorbance is measured on a microplate reader at 570 nm. Cell growth inhibition is evaluated as the ratio of the absorbance of the sample to that of the control. The results are representative of at least 3 independent experiments. |
| Animal Admin | Male nu/nu BALB/c mice are used in the assay. HL-60 cells are inoculated s.c. on the flanks of 4- to 6-wk-old mice. Tumors are measured every other day with use of calipers. Mice bearing HL-60 xenografts are randomized to receive treatment with normal saline (control) or p-niclosamide for 15 days (n=7 animals each). Tumor volumes are calculated by the following formula: a2×b×0.4, where a is the smallest diameter and b is the diameter perpendicular to a. After mice are euthanized, xenografts are dissected, weighed, or preserved. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 424.5±45.0 °C at 760 mmHg |
| Melting Point | 225-230ºC |
| Molecular Formula | C13H8Cl2N2O4 |
| Molecular Weight | 327.120 |
| Flash Point | 210.5±28.7 °C |
| Exact Mass | 325.986115 |
| PSA | 95.15000 |
| LogP | 5.41 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.709 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H400 |
| Precautionary Statements | P273 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xi |
| RIDADR | UN 3077 9/PG 3 |
| WGK Germany | 2 |
| RTECS | VN8400000 |
| Hazard Class | 9.0 |
| HS Code | 2924299090 |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |