70-00-8
| Name | trifluridine |
|---|---|
| Synonyms |
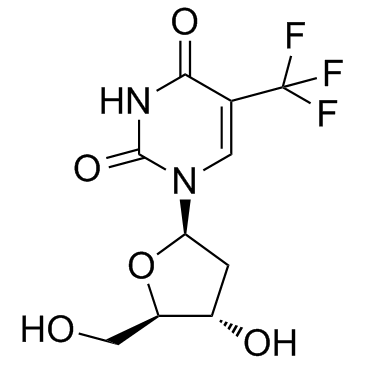
1-[(2R,4S,5R)-4-hydroxy-5-(hydroxyméthyl)tétrahydrofuran-2-yl]-5-(trifluorométhyl)pyrimidine-2,4(1H,3H)-dione
Viroptic α,α,α-Trifluorothymidine 2'-Deoxy-5-(trifluoromethyl)uridine UNII-RMW9V5RW38 Trifluorothymidine 1-(2-Deoxy-β-D-glycero-pentofuranosyl)-5-(trifluoromethyl)pyrimidine-2,4(1H,3H)-dione Trifluridine 1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-5-(trifluoromethyl)pyrimidine-2,4(1H,3H)-dione EINECS 200-722-8 1-[(2R,4S,5R)-4-Hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]-5-(trifluormethyl)pyrimidin-2,4(1H,3H)-dion 2'-Deoxy-5-trifluoromethyluridine Virophta a,a,a-Trifluorothymidine MFCD00006534 |
| Description | Trifluridine is an irreversible thymidylate synthase inhibitor, and thereby suppresses DNA synthesis. Trifluridine is an antiviral drug for herpes simplex virus (HSV) infection. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Melting Point | 190-193 °C(lit.) |
| Molecular Formula | C10H11F3N2O5 |
| Molecular Weight | 296.200 |
| Exact Mass | 296.062012 |
| PSA | 104.55000 |
| LogP | 0.07 |
| Index of Refraction | 1.534 |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 + H312 + H332-H351 |
| Precautionary Statements | P261-P280-P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R20/21/22;R40 |
| Safety Phrases | S22-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | XP2087500 |
| Precursor 8 | |
|---|---|
| DownStream 2 | |


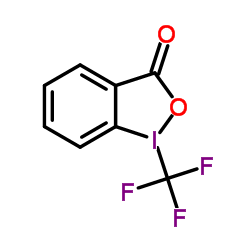
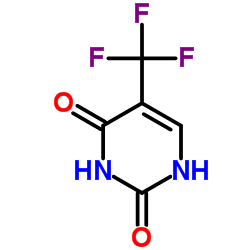
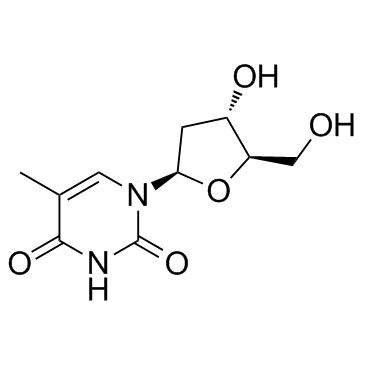
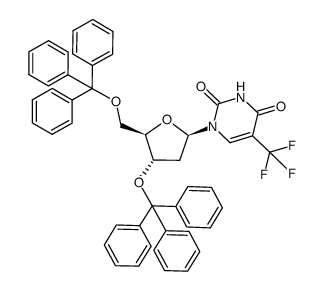
![[5-[2,4-dioxo-5-(trifluoromethyl)pyrimidin-1-yl]-3-(4-methylbenzoyl)oxy-oxolan-2-yl]methyl 4-methylbenzoate structure](https://image.chemsrc.com/caspic/066/7057-46-7.png)