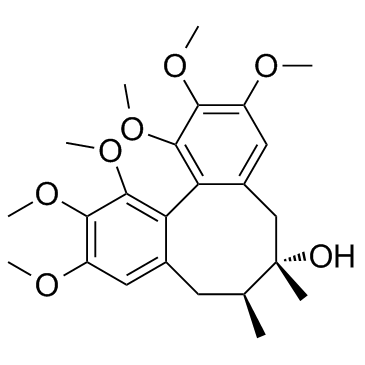
61281-38-7
| Name | Schisandrin A |
|---|---|
| Synonyms |
(-)-Deoxyschisandrin
(+)-Deoxyschisandrin Schizandrin A (+)-Dimethylgomisin J (6R,7S)-1,2,3,10,11,12-Hexamethoxy-6,7-dimethyl-5,6,7,8-tetrahydrodibenzo[a,c][8]annulene UNII:74XQL5DO3S Dimethylgomisin J (-)-Dimethylgomisin J DEOXYSCHISANDRIN SchisandrinA Doxyschizandrin |
| Description | Schisandrin A inhibits CYP3A activity with an IC50 of 6.60 μM and Ki of 5.83 μM, respectively. |
|---|---|
| Related Catalog | |
| Target |
CYP3A:6.6 μM (IC50) Autophagy |
| In Vitro | Schisandrin A (Sch A) strongly inhibits microsomal midazolam 1-hydroxylation catalyzed by CYP3A, with an IC50 of 6.60 μM. The recovery of enzyme activity in the absence or presence of Schisandrin A is shown in dilution assay plots. The Ki value for Schisandrin A is obtained from the Dixon plots and is 5.83 μM. The inactivation of rat liver microsomal midazolam 1-hydroxylation activity by Schisandrin A in the presence of NADPH is found to be time- and concentration-dependent. The Kinact and Ki are estimated to be 0.134/min and 4.51 μM, respectively for Schisandrin A[1]. |
| In Vivo | Schisandrin A (SchA) significantly inhibits CYP3A activity in rat hepatic microsomes and Vmax value of each group in a concentration-dependent manner. The double-reciprocal plots and the secondary plot show that Schisandrin A inhibits CYP3A activity, with an apparent Ki value of 30.67 mg/kg. In each Schisandrin A-treated group, Schisandrin A also significantly decreases 1-hydroxymidazolam plasma concentrations compared with the negative group (to levels similar to the positive group)[2]. |
| Kinase Assay | For the inactivation of CYP3A4 activity, microsomes are preincubated with inhibitors (Schisandrin A, 2.4 μM, 7.2 μM and 12.0 μM; or Sch B) at 37°C for up to 15 min in the presence of NADPH. Reactions are initiated with the addition of substrate midazolam and incubated at 37°C for 10 min. The enzyme inactivation is analyzed. Duplicates are prepared and tested[1]. |
| Animal Admin | Rats[2] Healthy male Sprague-Dawley rats, weighing 250-280 g and 2-3 months of age, are used. The rats are randomly divided into five groups with 16 rats in each group. The animals are administered once daily for three consecutive days. The Schisandrin A-treated groups are administered intragastrically with doses of 32, 16 or 8 mg/kg of Schisandrin A (physiological saline as vehicle), and the rats are similarly administered with equal volume of vehicle in the negative control group and Ketoconazole (75 mg/kg) in the positive control group. All animals are allowed free access to food but are fasted overnight before scarification to reduce the intestinal content, and each group is randomly divided into two parts with eight rats in each part[2]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 544.2±50.0 °C at 760 mmHg |
| Melting Point | 114 °C |
| Molecular Formula | C24H32O6 |
| Molecular Weight | 416.507 |
| Flash Point | 215.6±30.0 °C |
| Exact Mass | 416.219879 |
| PSA | 55.38000 |
| LogP | 5.87 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.520 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn |
|---|---|
| Safety Phrases | 24/25 |
| RIDADR | NONH for all modes of transport |
| RTECS | HP1595000 |
| HS Code | 2932999099 |
|
~% 
61281-38-7 |
| Literature: Chemical & Pharmaceutical Bulletin, , vol. 28, p. 2414 - 2421 |
|
~% 
61281-38-7 |
| Literature: Chemical & Pharmaceutical Bulletin, , vol. 28, p. 2414 - 2421 |
| Precursor 1 | |
|---|---|
| DownStream 1 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |