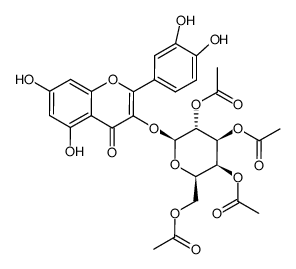
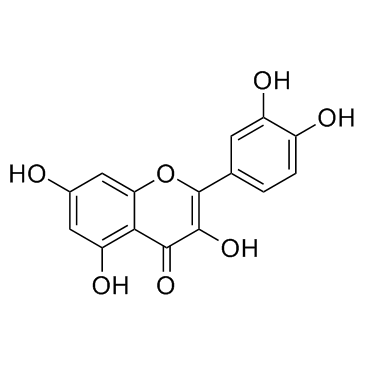
482-36-0
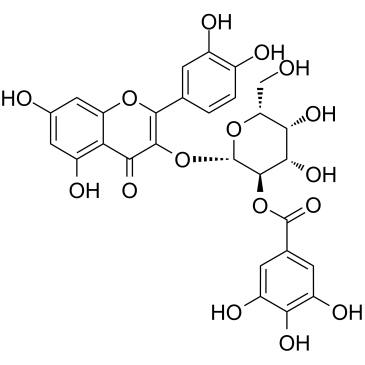
| Name | quercetin 3-O-β-D-galactopyranoside |
|---|---|
| Synonyms |
2-(3,4-Dihydroxyphenyl)-3-(β-D-galactopyranosyloxy)-5,7-dihydroxy-4H-1-benzopyran-4-one
Quercetin 3-O-β-D-galactoside quercetin-3-O-galactoside Hyperin Quercetin 3-O-galactoside Quercetin 3-β-D-galactopyranoside Hyperoside Quercetin 3-O-β-D-galactopyranoside Quercetin-3-O-β-D-galactoside MFCD00016933 Hyperasid Hyperosid quercetin-3-β-O-galactoside Quercetin 3-galactoside quercetin 3-O-beta-D-galactopyranoside 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl β-D-galactopyranoside hyperozide Quercetin 3-D-galactoside EINECS 207-580-6 quercetin-3-galactoside |
| Description | Hyperoside, a natural flavonoid, isolated from Camptotheca acuminate, possesses antifungal, anti-inflammatory, anti-viral, anti-oxidative and anti-apoptotic activities[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 872.6±65.0 °C at 760 mmHg |
| Melting Point | 225-226ºC |
| Molecular Formula | C21H20O12 |
| Molecular Weight | 464.376 |
| Flash Point | 307.5±27.8 °C |
| Exact Mass | 464.095490 |
| PSA | 210.51000 |
| LogP | 1.75 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.803 |
| Storage condition | -20°C Freezer, Under Inert Atmosphere |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | 22-45 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DJ3009200 |
|
~78% 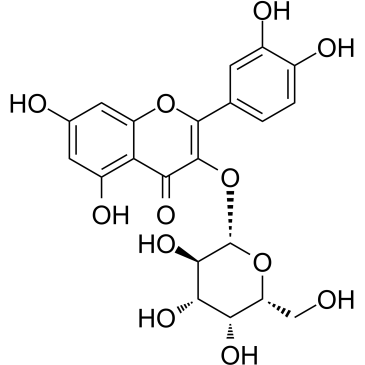
482-36-0 |
| Literature: Chen, Lili; Li, Jian; Luo, Cheng; Liu, Hong; Xu, Weijun; Chen, Gang; Liew, Oi Wah; Zhu, Weiliang; Puah, Chum Mok; Shen, Xu; Jiang, Hualiang Bioorganic and Medicinal Chemistry, 2006 , vol. 14, # 24 p. 8295 - 8306 |
|
~% 
482-36-0 |
| Literature: Chen, Zhiwei; Hu, Yongzhou; Wu, Haohao; Jiang, Huidi Bioorganic and Medicinal Chemistry Letters, 2004 , vol. 14, # 15 p. 3949 - 3952 |
|
~% 
482-36-0 |
| Literature: Chen, Zhiwei; Hu, Yongzhou; Wu, Haohao; Jiang, Huidi Bioorganic and Medicinal Chemistry Letters, 2004 , vol. 14, # 15 p. 3949 - 3952 |
|
~% 
482-36-0 |
| Literature: Chen, Zhiwei; Hu, Yongzhou; Wu, Haohao; Jiang, Huidi Bioorganic and Medicinal Chemistry Letters, 2004 , vol. 14, # 15 p. 3949 - 3952 |
|
~% 
482-36-0 |
| Literature: Yazaki, Kazufumi; Shida, Shoko; Okuda, Takuo Phytochemistry (Elsevier), 1989 , vol. 28, # 2 p. 607 - 610 |
|
~% 
482-36-0 |
| Literature: Masuda; Iritani; Yonemori; Oyama; Takeda Bioscience, biotechnology, and biochemistry, 2001 , vol. 65, # 6 p. 1302 - 1309 |
| Precursor 4 | |
|---|---|
| DownStream 4 | |