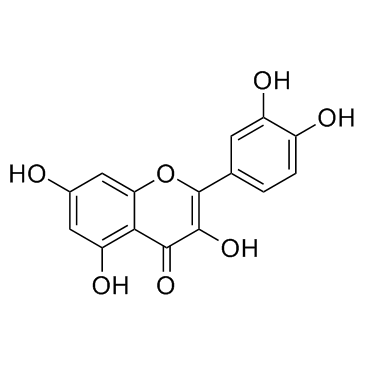
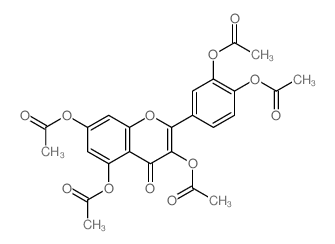
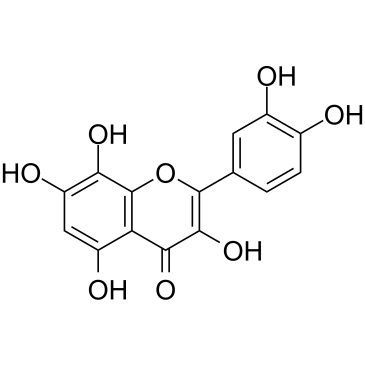
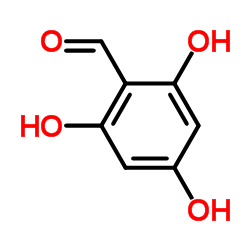
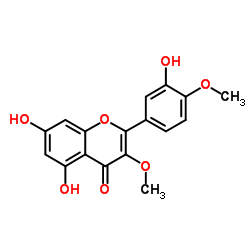
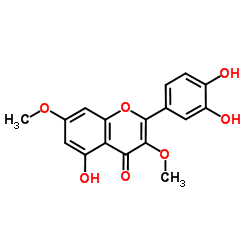
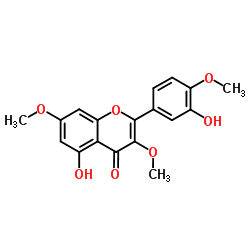
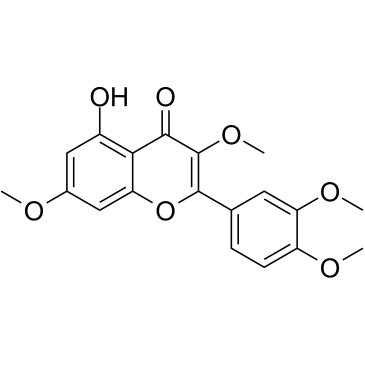
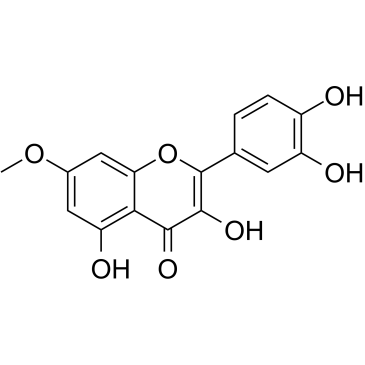
117-39-5
| Name | quercetin |
|---|---|
| Synonyms |
EINECS 204-187-1
MELETIN 2',3,4',5,7-pentahydroxyflavone MFCD00006828 QUERTINE 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one Sophretin QUERCETOL QUERCETINE SOPHORETIN 3,3',4',5,7-Pentahydroxyflavone Quercetin QUERCITIN kvercetin 3,3',4',5,7-pentahydroxylflavone |
| Description | Quercetin is a natural flavonoid which activates or inhibits the activities of a number of proteins. Quercetin can activate SIRT1 and inhibit PI3K with IC50s of 2.4 μM, 3.0 μM, 5.4 μM for PI3K γ, PI3K δ and PI3K β, respecti |
|---|---|
| Related Catalog | |
| Target |
PI3Kβ:5.4 μM (IC50) PI3Kδ:2.4 μM (IC50) PI3Kγ:3 μM (IC50) Autophagy Mitophagy |
| In Vitro | Quercetin is a type of plant-based chemical, or phytochemical, used as an ingredient in supplements, beverages or foods. In several studies, it may have anti-inflammatory and antioxidant properties, and it is being investigated for a wide range of potential health benefits. Quercetin is a PI3K inhibitor with IC50 of 2.4-5.4 μM. It strongly abrogates PI3K and Src kinases, mildly inhibits Akt1/2, and slightly affected PKC, p38 and ERK1/2[1]. Quercetin inhibits TNF-induced LDH% release, EC-dependent neutrophils adhesion to bovine pulmonary artery endothelial cells (BPAEC), and BPAEC DNA synthesis and proliferation[2]. |
| In Vivo | Combination of Quercetin (75 mg/kg) and 2-Methoxyestradiol enhances inhibition of human prostate cancer LNCaP and PC-3 cells xenograft tumor growth[3]. |
| Animal Admin | Mice are inoculated subcutaneously with 5×105 PC-3 cells suspended in 100μL PBS and 2×108 LNCaP cells suspended in 100μL of matrigel and PBS mixture (1:1) on the right back. When xenograft tumors reach a volume of approximately 100 mm3, mice are randomLy assigned to four groups (n=8 each group) and treated intraperitoneally. Therapeutic schedule based on our in vitro results, preliminary experiments and many other researchers' studies is as follows: (1) Vehicle control group: vehicle of quercetin on day 1, vehicle of 2-ME on day 2, (2) Quercetin treated group: quercetin 75 mg/kg on day 1, vehicle of 2-ME on day 2, (3) 2-ME treated group: vehicle of quercetin on day 1, 2-ME 150 mg/kg on day 2, (4) Combination treatment group: quercetin 75 mg/kg on day 1, 2-ME 150 mg/kg on day 2. Two days is a treatment cycle and the whole treatment process lasted for 4 weeks. Tumor sizes are monitored every 2 days using caliper and tumor volume are calculated according to the formula: L×S2×0.5, in which L represents the longest diameter and S represents the shortest diameter of tumor. Mice are weighed as well. At the end of treatment procedure, on day 29, mice are anesthetized with chloral hydrate and sacrificed by cervical dislocation. Xenograft tumors are taken out quickly and weighed. One part of it is put into liquid nitrogen immediately for future biomarker analysis and the other part is fixed in 10% neutral buffered formalin for immunohistochemical analysis. Serum biochemical parameters such as ALT, AST, creatinine and urea nitrogen that reflected drug toxicity are also detected. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 642.4±55.0 °C at 760 mmHg |
| Melting Point | 314-317°C |
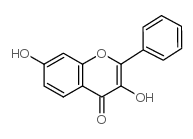
| Molecular Formula | C15H10O7 |
| Molecular Weight | 302.236 |
| Flash Point | 248.1±25.0 °C |
| Exact Mass | 302.042664 |
| PSA | 131.36000 |
| LogP | 2.08 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.823 |
| Storage condition | Store at 0-5°C |
| Water Solubility | <0.1 g/100 mL at 21 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 + P330 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R25;R40 |
| Safety Phrases | S45 |
| RIDADR | 2811 |
| RTECS | LK8750000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2932999099 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914501900 |
|---|---|
| Summary | 2914501900 other ketone-phenols。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |