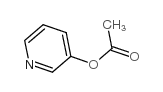
105462-24-6
| Name | Risedronic Acid |
|---|---|
| Synonyms |
Actonel
[1-Hydroxy-2-(3-pyridinyl)-1,1-ethanediyl]bis(phosphonic acid) (1-hydroxy-1-phosphono-2-pyridin-3-ylethyl)phosphonic acid (1-hydroxy-2-pyridin-3-ylethane-1,1-diyl)bis(phosphonic acid) MFCD00867080 T6NJ C1XQPQQO&PQQO [1-Hydroxy-2-(3-pyridinyl)ethylidene]bisphosphonic acid 2-(3-Pyridinyl)-1-hydroxyethanediphosphonic acid [1-Hydroxy-2-(3-pyridinyl)ethylidene]bis(phosphonic acid) Risedronic acid [1-Hydroxy-2-(pyridin-3-yl)ethane-1,1-diyl]bis(phosphonic acid) Atelvia |
| Description | Risedronic acid (Risedronate ) is a pyridinyl biphosphonate which inhibits osteoclast-mediated bone resorption.Target: OthersRisedronate, which was promoted in Croatia a few months ago, is the latest (III) generation of bisphosphonates, the most efficient anti-resorption drugs that inhibit osteoclast-mediated bone resorption and change the bone metabolism. Risedronate is hence the first line of bisphosphonates for the reduction of vertebral and non-vertebral fracture risks in postmenopausal women with osteoporosis or those with a high risk of osteoporosis. It also efficiently prevents bone loss or improves bone density in men and women on a long-term corticosteroid therapy .The administration of 20 and 25 mg/kg risedronate for 4 days led to decreases of parasitemia of 68.9% and 83.6%, respectively. On the seventh day of treatment the inhibitions were 63% and 88.9% with 20 and 25 mg/kg, respectively. After recovering the parasitemia, a dose-response curve was obtained for estimating the ID50 (dose causing 50% inhibition), equivalent to 17 ± 1.8 mg/kg after 7 days of treatment. Four days after the interruption of treatment (11 days postinfection), the parasitemias of the groups treated with 10, 15, 20, and 25 mg/kg/day were 15.3%, 15.9%, 15.2%, and 5.7%, respectively. Conversely, the group that received PBS presented parasitemia of 25.6%. Among the groups treated with risedronate, only the animals that received 25 mg/kg had a significant inhibition of 77.8% (see Table S1 in the supplemental material), demonstrating that even after treatment discontinuation, the parasitemia of the animals remained low in relation to that of the controls . |
|---|---|
| Related Catalog | |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 692.3±65.0 °C at 760 mmHg |
| Molecular Formula | C7H11NO7P2 |
| Molecular Weight | 283.112 |
| Flash Point | 372.5±34.3 °C |
| Exact Mass | 283.001068 |
| PSA | 167.80000 |
| LogP | -2.94 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.651 |
| Storage condition | 2~8°C |
| Hazard Codes | Xi |
|---|---|
| WGK Germany | 3.0 |
| HS Code | 2933399090 |
|
~98% 
105462-24-6 |
| Literature: JUBILANT ORGANOSYS LIMITED Patent: WO2006/51553 A1, 2006 ; Location in patent: Page/Page column 8 ; |
|
~81% 
105462-24-6 |
| Literature: FLEMING LABORATORIES LIMITED Patent: WO2009/34580 A1, 2009 ; Location in patent: Page/Page column 7 ; |
|
~82% 
105462-24-6 |
| Literature: ZAKLADY FARMACEUTYCZNE S.A.; POLITECHNIKA GDANSKA Patent: WO2006/71128 A1, 2006 ; Location in patent: Page/Page column 4; 5 ; |
|
~% 
105462-24-6 |
| Literature: WO2008/58722 A1, ; Page/Page column 11-12 ; |
|
~% 
105462-24-6 |
| Literature: WO2008/44245 A2, ; Page/Page column 5 ; |
|
~% 
105462-24-6 |
| Literature: US5391743 A1, ; |
|
~% 
105462-24-6 |
| Literature: Phosphorus, Sulfur and Silicon and the Related Elements, , vol. 188, # 1-3 p. 39 - 41 |
| Precursor 6 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |