1257256-44-2
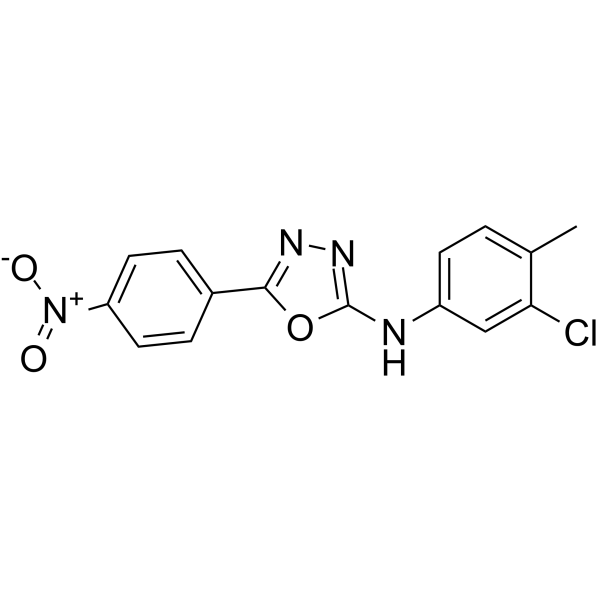
| Name | N-(3-Chloro-4-methylphenyl)-5-(4-nitrophenyl)-1,3,4-oxadiazol-2-a mine |
|---|---|
| Synonyms |
3-Pyridinecarboxamide,N-(3-chloro-4-isoquinolinyl)-2-(cyclopropylamino)
N-(3-CHLOROISOQUINOLIN-4-YL)-2-CYCLOPROPYLAMINONICOTINAMIDE N-(3-chloro-4-methylphenyl)-5-(4-nitrophenyl)-1,3,4-oxadiazol-2-amine N-(3-chloro-4-methyl-phenyl)-3-methyl-benzamide N-(3-chloro-4-isoquinolinyl)-2-cyclopropylamino-3-pyridinecarboxamide |
| Description | TC-G 24 (Compound 24) is a potent, selective glycogen synthase kinase-3β (GSK-3β) inhibitor with an IC50 of 17.1 nM. TC-G 24 can cross the BBB and can be used for studying many diseases such as type 2 diabetes mellitus, stroke, Alzheimer, and other related diseases[1]. |
|---|---|
| Related Catalog | |
| Target |
GSK-3β:17.1 nM (IC50) |
| In Vitro | TC-G 24 (Compound 24) binds to the ATP binding site of GSK-3β[1]. TC-G 24 (1 µM, 4 h) blocks the FBW7α-mediated degradation of TPP1 in human embryonic kidney (HEK) 293T cells[2]. Western Blot Analysis[2] Cell Line: 293T cells Concentration: 1 μM Incubation Time: 4 h Result: Blocked the FBW7α-mediated degradation of TPP1 |
| In Vivo | TC-G 24 (Compound 24) (0-15 mg/kg; i.p.; once) significantly raises liver glycogen content in a dose-dependent manner without obvious toxicity and can cross the BBB[1]. Animal Model: Six-week-old male C57BL/6N mice with weights averaging 22 g[1] Dosage: 1, 5, and 15 mg/kg Administration: Intraperitoneal injection, once Result: Significantly raised liver glycogen content in a dose-dependent manner without obvious toxicity. Was detected in the brain at concentrations higher than in plasma for all three tested doses (38 ± 6, 113 ± 54 and 286 ± 58 ng/g brain tissue at 1, 5 and 15 mg/kg, respectively). |
| References |
| Molecular Formula | C15H11ClN4O3 |
|---|---|
| Molecular Weight | 330.73 |
| Exact Mass | 330.05200 |
| PSA | 100.00000 |
| LogP | 4.29530 |