573-40-0
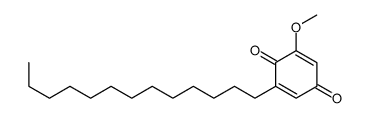
| Name | 2,5-dihydroxy-3-tridecylcyclohexa-2,5-diene-1,4-dione |
|---|---|
| Synonyms |
Rapanone
2,4-DIHYDRO-4-PHENYL-3H-1,2,4-TRIAZOL-3-ONE 2,5-dihydroxy-3-tridecyl-1,4-benzoquinone 2,5-Dihydroxy-3-tridecyl-benzo<1,4>chinon |
| Description | Rapanone is a natural benzoquinone. Rapanone exhibits a broad spectrum of biological actions, including anti-tumor, antioxidant, anti-inflammatory, antibacterial and antiparasitic. Rapanone also is a potent and selective human synovial PLA2 inhibitor, with an IC50 of 2.6 μM[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
PLA2:2.6 μM (IC50) |
| In Vitro | Rapanone (10-40 μM; 24 h) inhibits the cell viability, with IC50s of 35.58 μM and 27.89 μM for primary rats hepatocytes and HepG2 cells, respectively[1]. Rapanone (10-40 μM; 24 h) induces a concentration-dependent mitochondrial membrane potential dissipation, ATP depletion, hydrogen peroxide generation and, phosphatidyl serine externalization in HepG2 cells[1]. Rapanone inhibits electron transport at Complex III and promotes mitochondrial dysfunction[1]. |
| In Vivo | Rapanone (2.5-10 mg/kg; i.p.) exhibits anti-inflammatory effects in the carrageenan paw oedema model in mice[4]. |
| References |
| Density | 1.099g/cm3 |
|---|---|
| Boiling Point | 457ºC at 760mmHg |
| Melting Point | 142-145ºC |
| Molecular Formula | C19H30O4 |
| Molecular Weight | 322.43900 |
| Flash Point | 244.3ºC |
| Exact Mass | 322.21400 |
| PSA | 74.60000 |
| LogP | 5.09330 |
| Index of Refraction | 1.53 |
| HS Code | 2914690090 |
|---|
|
~89% 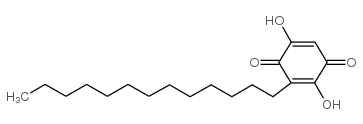
573-40-0 |
| Literature: McErlean, Christopher S. P.; Moody, Christopher J. Journal of Organic Chemistry, 2007 , vol. 72, # 26 p. 10298 - 10301 |
|
~% 
573-40-0 |
| Literature: Fieser; Chamberlin Journal of the American Chemical Society, 1948 , vol. 70, p. 74 |
|
~% 
573-40-0 |
| Literature: Croft,J.A. et al. Australian Journal of Chemistry, 1976 , vol. 29, p. 1979 - 1987 |
|
~% 
573-40-0 |
| Literature: Croft,J.A. et al. Australian Journal of Chemistry, 1976 , vol. 29, p. 1979 - 1987 |
|
~% 
573-40-0 |
| Literature: Croft,J.A. et al. Australian Journal of Chemistry, 1976 , vol. 29, p. 1979 - 1987 |
|
~% 
573-40-0 |
| Literature: Maruyama,K. et al. Journal of Organic Chemistry, 1978 , vol. 43, # 25 p. 4895 - 4898 |
|
~% 
573-40-0 |
| Literature: Maruyama,K. et al. Journal of Organic Chemistry, 1978 , vol. 43, # 25 p. 4895 - 4898 |
|
~% 
573-40-0 |
| Literature: Asano; Yamaguchi Yakugaku Zasshi, 1940 , vol. 60, p. 585,587, 591; engl. Ref. S. 237, 239, 242 Chem.Abstr., 1942 , p. 81 |
| Precursor 7 | |
|---|---|
| DownStream 4 | |
| HS Code | 2914690090 |
|---|---|
| Summary | 2914690090 other quinones。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |