530-59-6
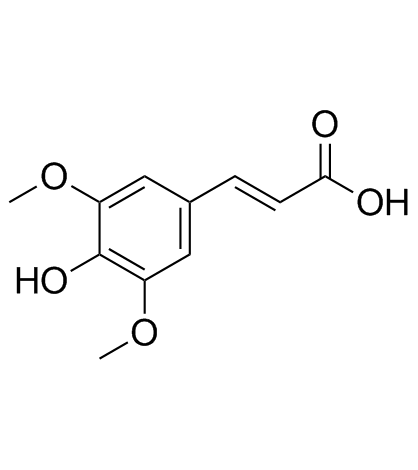
| Name | trans-sinapic acid |
|---|---|
| Synonyms |
4-Hydroxy-3,5-dimethoxycinnamic acid
Sinapic acid 3,5-Dimethoxy-4-hydroxycinnamic Acid 3-(4-Hydroxy-3,5-dimethoxyphenyl)acrylic acid 3 5-dimethoxy-4-hydroxycinnamic acid (2E)-3-(4-Hydroxy-3,5-dimethoxyphenyl)acrylic acid (2E)-3-(4-hydroxy-3,5-dimethoxyphenyl)prop-2-enoic acid Sinapinic acid 3,5-DIMETHOXY-4-HYDROXY-TRANS-CINNAMIC ACID (E)-sinapic acid EINECS 208-487-3 MFCD00004401 trans-3,5-Dimethoxy-4-hydroxycinnamic Acid trans-sinapic acid |
| Description | Sinapinic acid (Sinapic acid) is a phenolic compound isolated from Hydnophytum formicarum Jack. Rhizome, acts as an inhibitor of HDAC, with an IC50 of 2.27 mM[1], and also inhibits ACE-I activity[2]. Sinapinic acid posssess potent anti-tumor activity, induces apoptosis of tumor cells[1]. Sinapinic acid shows antioxidant and antidiabetic activities[2]. Sinapinic acid reduces total cholesterol, triglyceride, and HOMA-IR index, and also normalizes some serum parameters of antioxidative abilities and oxidative damage in ovariectomized rats[3]. |
|---|---|
| Related Catalog | |
| Target |
HDAC:2.27 mM (IC50) ACE-I |
| In Vitro | Sinapinic acid acts as an inhibitor of HDAC, with an IC50 of 2.27 mM[1]. Sinapinic acid also inhibits ACE-I activity[2]. Sinapinic acid inhibits HDAC activity in HeLa cells, suppresses the growth of HeLa and HT29 cells with IC50s of 0.91 ± 0.02 mM and 1.6 ± 0.02 mM at 72 h, respectively, induces apoptosis of these cancer cells[1]. |
| In Vivo | Sinapinic acid (5 or 25 mg/kg, p.o. daily for 4 weeks) increases the serum estradiol concentration; decreases insulin resistance and the triglyceride and total cholesterol concentrations; and favorably affects the parameters of antioxidant abilities (reduces glutathione, superoxide dismutase) and oxidative damage in rats[3]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 403.4±40.0 °C at 760 mmHg |
| Melting Point | 203-205 °C (dec.)(lit.) |
| Molecular Formula | C11H12O5 |
| Molecular Weight | 224.210 |
| Flash Point | 158.6±20.8 °C |
| Exact Mass | 224.068466 |
| PSA | 75.99000 |
| LogP | 1.29 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.604 |
| Stability | Stable. Incompatible with strong oxidizing agents, strong bases. Combustible. |
| Water Solubility | insoluble |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2918990090 |
|
~% 
530-59-6 |
| Literature: Journal of the American Chemical Society, , vol. 70, p. 57 |
|
~% 
530-59-6 |
| Literature: EP1604643 A1, ; Page/Page column 10-11 ; |
|
~72% 
530-59-6 |
| Literature: Chimichi, Stefano; Sio, Francesco De; Donati, Donato; Fina, Giuseppe; Pepino, Roberto; Sarti-Fantoni, Piero Heterocycles, 1983 , vol. 20, # 2 p. 263 - 267 |
|
~% 
530-59-6 |
| Literature: Phytochemistry (Elsevier), , vol. 12, p. 893 - 897 |
|
~% 
530-59-6 |
| Literature: Chemistry and Biodiversity, , vol. 9, # 1 p. 91 - 98 |
|
~% 
530-59-6 |
| Literature: Phytochemistry (Elsevier), , vol. 29, # 9 p. 2999 - 3001 |
|
~% 
530-59-6 |
| Literature: Heterocycles, , vol. 20, # 2 p. 263 - 267 |
|
~% 
530-59-6 |
| Literature: Journal of the Chinese Chemical Society, , vol. 56, # 6 p. 1186 - 1190 |
| HS Code | 2918990090 |
|---|---|
| Summary | 2918990090. other carboxylic acids with additional oxygen function and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |