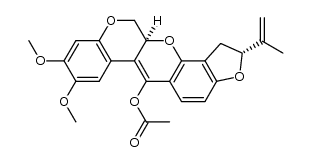
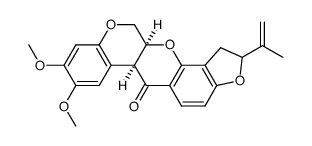
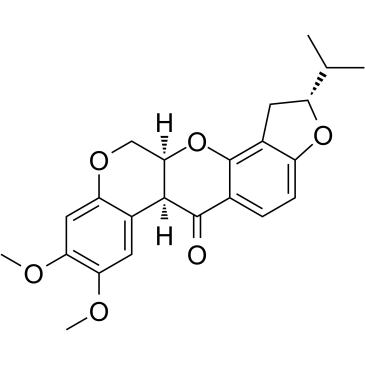
83-79-4
| Name | rotenone |
|---|---|
| Synonyms |
(-)-rotenone
derris (2R,6aS,12aS)-8,9-dimethoxy-2-(1-methylethenyl)-1,2,12,12a-tetrahydrochromeno[3,4-b]furo[2,3-h]chromen-6(6aH)-one protax (2R,6aS,12aS)-8,9-Dimethoxy-2-(prop-1-en-2-yl)-1,2,12,12a-tetrahydrochromeno[3,4-b]furo[2,3-h]chromen-6(6aH)-one Derris root [2R-(2a,6aa,12aa)]-1,2,12,12a-Tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)-[1]benzopyrano[3,4-b]furo[2,3-h][1]benzopyran-6(6aH)-one Rotacide E.C. (2R,6aS,12aS)-1,2,6,6a,12,12a-hexahydro-2-isopropenyl-8,9-dimethoxychromeno[3,4-b]furo[2,3-h]chromen-6-one MFCD09025614 5'-b-Rotenone TUBATOXIN (2R,6aS,12aS)-1,2,12,12a-tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)[1]benzopyrano[3,4-b]furo[2,3-h][1]benzopyran-6(6aH)-one Foliafume E.C. paraderil Dermostatin A Rotenone,(2R,6aS,12aS)-1,2,12,12a-Tetrahydro-8,9-dimethoxy-2-(1-methylethenyl)-[1]benzopyrano[3,4-b]furo[2,3-h][1]benzopyran-6(6aH)-one (-)-cis-rotenone (2R,6aS,12aS)-2-Isopropenyl-8,9-dimethoxy-1,2,12,12a-tetrahydrochromeno[3,4-b]furo[2,3-h]chromen-6(6aH)-one Noxfire EINECS 201-501-9 Tubotoxine Canex |
| Description | Rotenone is an mitochondrial electron transport chain complex I inhibitor. |
|---|---|
| Related Catalog | |
| In Vitro | Mitogen Activated Protein Kinase (MAPK), Toll-like receptor, Wnt, and Ras signaling pathways are intensively involved in the effect of rotenone on the ENS[2]. Rotenone-induced cell death is reduced by treatment as measured by decline in the levels of pro-apoptotic proteins. Moreover, treatment significantly augments the levels of anti-apoptotic Bcl2 and blocks the release of cytochrome c, thereby alleviating the rotenone-induced dopaminergic neuronal loss, as evidenced by tyrosine hydroxylase (TH) immunostaining in the striatum[3]. |
| In Vivo | Rotenone causes a significant increase in the excitatory amino acid neurotransmitters; glutamate and aspartate together with a significant decrease in the inhibitory amino acids, GABA, glycine and taurine are observed in the cerebellum of rat model of PD[1]. Rotenone (1.5, 2, or 2.5 mg/kg) causes a dose-dependent increase in α-synuclein in the substantia nigra. Furthermore, at 2 and 2.5 mg/kg, rotenone causes a significant decrease in the number of tyrosine hydroxylase-immunoreactive neurons in the substantia nigra, and dopamine in the striatum in rats[4]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 559.8±50.0 °C at 760 mmHg |
| Melting Point | 159-164 °C(lit.) |
| Molecular Formula | C23H22O6 |
| Molecular Weight | 394.417 |
| Flash Point | 244.6±30.2 °C |
| Exact Mass | 394.141632 |
| PSA | 63.22000 |
| LogP | 4.65 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.591 |
| Stability | Stable, but light and air sensitive. Combustible. Incompatible with oxidizing agents, especially in the presence of alkalies. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS06, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H315-H319-H335-H410 |
| Precautionary Statements | P273-P280-P301 + P310 + P330-P337 + P313-P391-P501 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic;N:Dangerousfortheenvironment; |
| Risk Phrases | R25;R36/37/38;R50/53 |
| Safety Phrases | 22-24/25-36-45-60-61 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | DJ2800000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
|
~81% 
83-79-4 |
| Literature: Bhandari, Prabha; Crombie, Leslie; Kilbee, Geoffrey W.; Pegg, Stephen J.; Proudfoot, Geoffrey; et al. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1992 , # 7 p. 851 - 864 |
|
~% 
83-79-4 |
| Literature: Singhal, Ashok Kumar; Sharma, Ram Prakash; Baruah, Jogendra Nath; Govindan, Serengolam V.; Herz, Werner Phytochemistry (Elsevier), 1982 , vol. 21, # 4 p. 949 - 951 |
|
~% 
83-79-4 |
| Literature: Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), , # 21 p. 2605 - 2614 |
|
~59% 
83-79-4 |
| Literature: Carson, David; Crombie, Leslie; Kilbee, Geoffrey W.; Moffatt, Frank; Whiting, Donald A. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1982 , p. 779 - 788 |
|
~% 
83-79-4 |
| Literature: Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), , # 21 p. 2605 - 2614 |
|
~% 
83-79-4 |
| Literature: Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), , # 7 p. 851 - 864 |
|
~% 
83-79-4 |
| Literature: Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), , p. 204 - 205 |
|
~% 
83-79-4 |
| Literature: Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), , # 7 p. 851 - 864 |
| Precursor 5 | |
|---|---|
| DownStream 5 | |