2141-09-5
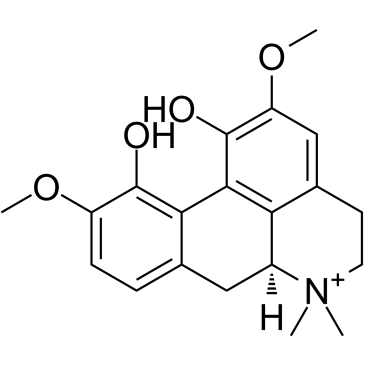
| Name | (+)-magnoflorine iodide |
|---|---|
| Synonyms |
Magflorine
escholin Magnoflorine Aporphine alkaloid escholine (6aS)-1,11-Dihydroxy-2,10-dimethoxy-6,6-dimethyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinolinium Thalictrine UNII-NI8K6962K4 THALICTRIN (S)-5,6,6a,7-Tetrahydro-1,11-dihydroxy-2,10-dimethoxy-6,6-dimethyl-4H-dibenzo[de,g]quinolinium Esholine |
| Description | (+)-Magnoflorine (Magnoflorine) is an aporphine alkaloid found in Acoruscalamus, with anti-fungal activity, reduces the formation of C. albicans’ biofilm[1]. Anti-antidiabeticand anti-oxidative activity[2]. |
|---|---|
| Related Catalog | |
| Target |
Fungal[1] |
| References |
| Melting Point | 252ºC |
|---|---|
| Molecular Formula | C20H24NO4+ |
| Molecular Weight | 342.408 |
| Exact Mass | 342.169983 |
| PSA | 58.92000 |
| LogP | -1.71 |
| Storage condition | 2-8C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 + H312 + H332 |
| Precautionary Statements | P261-P280-P301 + P312 + P330-P302 + P352 + P312-P304 + P340 + P312 |
| RIDADR | NONH for all modes of transport |
| RTECS | HQ1777600 |