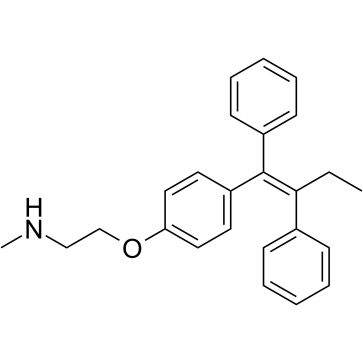
31750-48-8
| Name | n-desmethyltamoxifen, hydrochloride |
|---|---|
| Synonyms |
desmethyl Tamoxifen
N-Desmethyl-psilocybin N-demethyltamoxifen N-desmethyl-tamoxifen Baeocystin |
| Description | N-Desmethyltamoxifen is the major metabolite of tamoxifen in humans. N-Desmethyltamoxifen, a poor antiestrogen, is a ten-fold more potent protein kinase C (PKC) inhibitor than Tamoxifen. N-Desmethyltamoxifen is also a potent regulator of ceramide metabolism in human AML cells, limiting ceramide glycosylation, hydrolysis, and sphingosine phosphorylation[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
PKC |
| In Vitro | N-desmethyltamoxifen (20-500 ng/ml; 48 hours) has a profound inhibitory effect upon all seven glioma lines (T98G, U87, U138, U373, ALW, AUK, CAS cells)[1]. N-desmethyltamoxifen (1.5-10 μM; 114 hours) inhibits growth of MCF 7 human mammary carcinoma cells[2]. N-desmethyltamoxifen, resulting from the CYP3A4/5-mediated catalysis of tamoxifen, is the major primary quantitative metabolite of tamoxifen[3]. Cell Viability Assay[2] Cell Line: MCF 7 human mammary carcinoma cells Concentration: 1.5, 2.5, 5, 7.5, 10 μM Incubation Time: 114 hours Result: Inhibits growth of MCF 7 human mammary carcinoma cells |
| References |
| Density | 1.047 g/cm3 |
|---|---|
| Boiling Point | 485.8ºC at 760 mmHg |
| Molecular Formula | C25H27NO |
| Molecular Weight | 357.48800 |
| Flash Point | 213.2ºC |
| Exact Mass | 357.20900 |
| PSA | 21.26000 |
| LogP | 6.04480 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn |
|---|