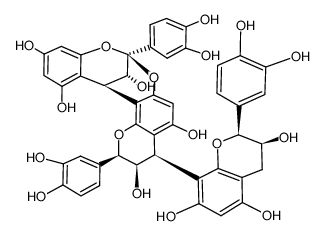
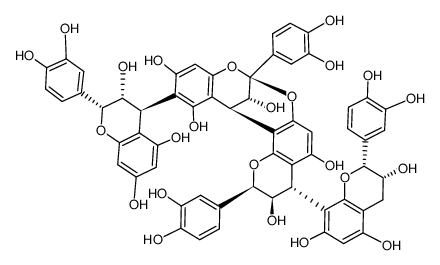
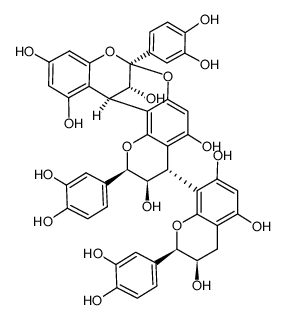
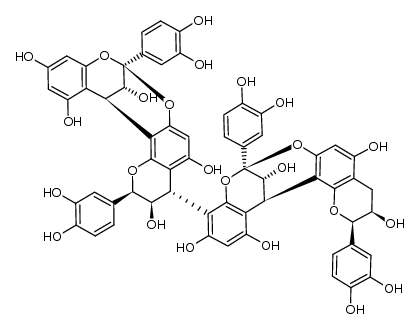
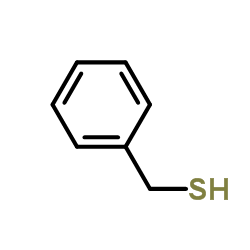
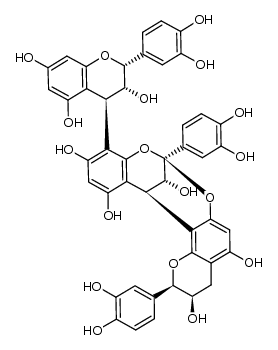
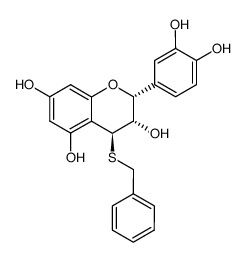
41743-41-3
| Name | proanthocyanidin A2 |
|---|---|
| Synonyms |
Procyanidin A2
7,4beta-&Procyanidol A2 8)-epicatechin (2r,3r,8s,14r,15r)-2,8-bis(3,4-dihydroxyphenyl)-3,4-dihydro-2h,14h-8,14-methanochromeno[7,8-d][1,3]benzodioxocine-3,5,11,13,15-pentol Proanthocyanidin A-2 Proanthocyanidin A2 Epicatechin-(2beta-&Procyanidin dimer A2 Dimeric catechin |
| Description | Procyanidin A2 is a flavonoid found in cranberries and lingonberries, with anti-cancer, antioxidant, antimicrobial and anti-inflammation activity[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Bacterial[1] |
| In Vitro | Procyanidin A2 shows DPPH radical scavenging activity, with an IC50 of 5.08 ± 0.37 μM[1]. |
| References |
| Density | 1.766g/cm3 |
|---|---|
| Boiling Point | 946ºC at 760 mmHg |
| Molecular Formula | C30H24O12 |
| Molecular Weight | 576.50 |
| Flash Point | 525.9ºC |
| Exact Mass | 576.12700 |
| PSA | 209.76000 |
| LogP | 2.79350 |
| Index of Refraction | 1.829 |
| Storage condition | 2-8°C |
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
|
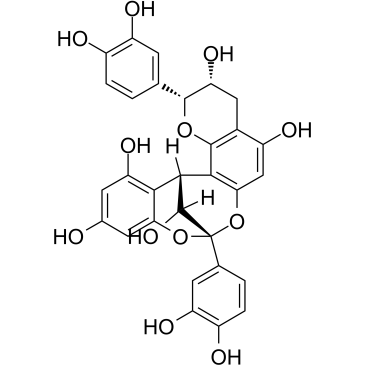
~% 
41743-41-3 |
| Literature: Nonaka, Gen-ichiro; Morimoto, Satoshi; Nishioka, Itsuo Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1983 , p. 2139 - 2145 |
|
~% 
41743-41-3 |
| Literature: Balde, A. M.; Pieters, L. A.; Wray, V.; Kolodziej, H.; Berghe, D. A. Vanden; et al. Phytochemistry (Elsevier), 1991 , vol. 30, # 12 p. 4129 - 4136 |
|
~% 
41743-41-3 |
| Literature: Balde, A. M.; Pieters, L. A.; Wray, V.; Kolodziej, H.; Berghe, D. A. Vanden; et al. Phytochemistry (Elsevier), 1991 , vol. 30, # 12 p. 4129 - 4136 |
|
~% 
41743-41-3 |
| Literature: Kondo, Kazunari; Kurihara, Masaaki; Fukuhara, Kiyoshi; Tanaka, Takashi; Suzuki, Takashi; Miyata, Naoki; Toyoda, Masatake Tetrahedron Letters, 2000 , vol. 41, # 4 p. 485 - 488 |
|
~% 
41743-41-3 |
| Literature: Nonaka, Gen-ichiro; Morimoto, Satoshi; Nishioka, Itsuo Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1983 , p. 2139 - 2145 |
|
~% 
41743-41-3 |
| Literature: Nonaka, Gen-ichiro; Morimoto, Satoshi; Nishioka, Itsuo Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1983 , p. 2139 - 2145 |
|
~% 
41743-41-3 |
| Literature: Morimoto, Satoshi; Nonaka, Gen-ichiro; Nishioka, Itsuo Chemical & Pharmaceutical Bulletin, 1987 , vol. 35, # 12 p. 4717 - 4729 |
|
~% 
41743-41-3 |
| Literature: Morimoto, Satoshi; Nonaka, Gen-ichiro; Nishioka, Itsuo Chemical & Pharmaceutical Bulletin, 1987 , vol. 35, # 12 p. 4717 - 4729 |
| Precursor 6 | |
|---|---|
| DownStream 0 | |