87-52-5
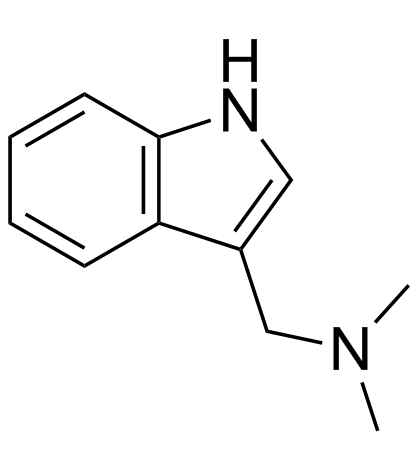
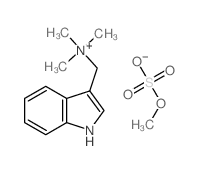
| Name | 1-(1H-indol-3-yl)-N,N-dimethylmethanamine |
|---|---|
| Synonyms |
Gramin
Donaxine Indole,3-[(dimethylamino)methyl] Gramine MFCD00005629 3-(Dimethylaminomethyl)indole Indol-3-ylmethyldimethylamine EINECS 201-749-8 Donaxin N,N-dimethyl-1H-indole-3-methanamine 3-(N,N-dimethylaminomethyl) indole N-(1H-indol-3-ylmethyl)-N,N-dimethylamine 1H-Indole-3-methanamine,N,N-dimethyl 1-(1H-Indol-3-yl)-N,N-dimethylmethanamine 3-[(Dimethylamino)methyl]indole (indol-3-ylmethyl)dimethylamine |
| Description | Gramine (Donaxine) is a natural alkaloid isolated from giant reed[2], acts as an active adiponectin receptor (AdipoR) agonist, with IC50s of 3.2 and 4.2 µM for AdipoR2 and AdipoR1, respectively[1]. Gramine is also a human and mouse β2-Adrenergic receptor (β2-AR) agonist[2]. Gramine (Donaxine) has anti-tumor, anti-viral and anti-inflammatory properties[1]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 3.2 µM (AdipoR2), 4.2 µM (AdipoR1)[1] |
| In Vitro | Gramine is an active adiponectin receptor (AdipoR) agonist, with IC50s of 3.2 and 4.2 µM for AdipoR2 and AdipoR1, respectively[1]. Gramine is a potential β2-AR agonist[2]. Gramine (20 µM to 1.2 nM) dose-dependently inhibits the growth of AdipoR1/adipoR2-positive cancer cell lines (MDA-MB-231 and MCF-7 cells), with IC50s of 9.6±0.9 and 0.1±0.1 µM, respectively[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 293.9±15.0 °C at 760 mmHg |
| Melting Point | 132-134 °C(lit.) |
| Molecular Formula | C11H14N2 |
| Molecular Weight | 174.242 |
| Flash Point | 131.5±20.4 °C |
| Exact Mass | 174.115692 |
| PSA | 19.03000 |
| LogP | 1.90 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.631 |
| Storage condition | Refrigerator (+4°C) |
| Water Solubility | PRACTICALLY INSOLUBLE |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi:Irritant; |
|---|---|
| Risk Phrases | R36 |
| Safety Phrases | S45-S36/37/39-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | NL7525000 |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |