|
![32502-63-9 structure]()
32502-63-9
|
|
Literature: Nagamatsu, Tomohisa; Yamasaki, Hirofumi Heterocycles, 1997 , vol. 45, # 4 p. 643 - 650 Title/Abstract Full Text View citing articles Show Details Nagamatsu, Tomohisa; Yamasaki, Hirofumi Journal of the Chemical Society. Perkin Transactions 1, 2001 , # 2 p. 130 - 137
|
![32502-63-9 structure]()
32502-63-9
|
|
Literature: Raoof, Ali; Depledge, Paul; Hamilton, Niall M.; Hamilton, Nicola S.; Hitchin, James R.; Hopkins, Gemma V.; Jordan, Allan M.; Maguire, Laura A.; McGonagle, Alison E.; Mould, Daniel P.; Rushbrooke, Mathew; Small, Helen F.; Smith, Kate M.; Thomson, Graeme J.; Turlais, Fabrice; Waddell, Ian D.; Waszkowycz, Bohdan; Watson, Amanda J.; Ogilvie, Donald J. Journal of Medicinal Chemistry, 2013 , vol. 56, # 16 p. 6352 - 6370
|
![32502-63-9 structure]()
32502-63-9
|
|
Literature: Nagamatsu, Tomohisa; Yamasaki, Hirofumi Heterocycles, 1997 , vol. 45, # 4 p. 643 - 650
|
![32502-63-9 structure]()
32502-63-9
|
|
Literature: Nagamatsu, Tomohisa; Yamasaki, Hirofumi Heterocycles, 1997 , vol. 45, # 4 p. 643 - 650
|
![32502-63-9 structure]()
32502-63-9
|
|
Literature: Nagamatsu, Tomohisa; Yamasaki, Hirofumi Heterocycles, 1997 , vol. 45, # 4 p. 643 - 650
|
![32502-63-9 structure]()
32502-63-9
|
|
Literature: Nagamatsu, Tomohisa; Yamasaki, Hirofumi Heterocycles, 1997 , vol. 45, # 4 p. 643 - 650
|
![32502-63-9 structure]()
32502-63-9
|
|
Literature: Raoof, Ali; Depledge, Paul; Hamilton, Niall M.; Hamilton, Nicola S.; Hitchin, James R.; Hopkins, Gemma V.; Jordan, Allan M.; Maguire, Laura A.; McGonagle, Alison E.; Mould, Daniel P.; Rushbrooke, Mathew; Small, Helen F.; Smith, Kate M.; Thomson, Graeme J.; Turlais, Fabrice; Waddell, Ian D.; Waszkowycz, Bohdan; Watson, Amanda J.; Ogilvie, Donald J. Journal of Medicinal Chemistry, 2013 , vol. 56, # 16 p. 6352 - 6370
|
|
![32502-63-9 structure]()
32502-63-9
|
|
Literature: Raoof, Ali; Depledge, Paul; Hamilton, Niall M.; Hamilton, Nicola S.; Hitchin, James R.; Hopkins, Gemma V.; Jordan, Allan M.; Maguire, Laura A.; McGonagle, Alison E.; Mould, Daniel P.; Rushbrooke, Mathew; Small, Helen F.; Smith, Kate M.; Thomson, Graeme J.; Turlais, Fabrice; Waddell, Ian D.; Waszkowycz, Bohdan; Watson, Amanda J.; Ogilvie, Donald J. Journal of Medicinal Chemistry, 2013 , vol. 56, # 16 p. 6352 - 6370
|
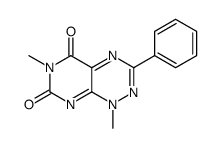
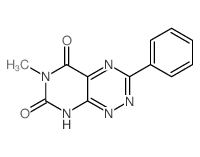


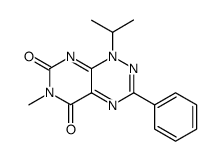
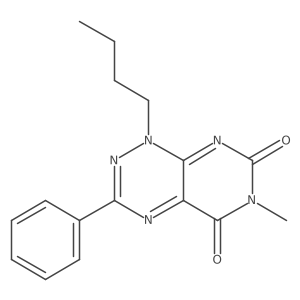
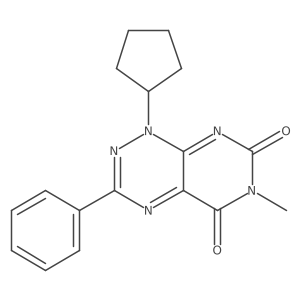
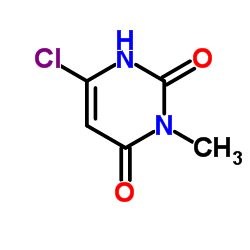

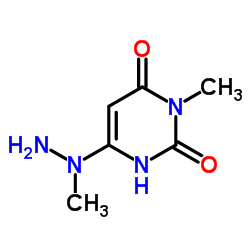
![Pyrimido[5,4-e]-1,2,4-triazine-5,7(6H,8H)-dione,6,8-dimethyl-3-phenyl structure](https://image.chemsrc.com/caspic/234/25696-85-9.png)