N-Acetyltryptamine
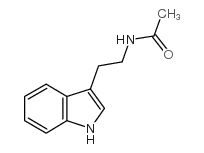
N-Acetyltryptamine structure
|
Common Name | N-Acetyltryptamine | ||
|---|---|---|---|---|
| CAS Number | 1016-47-3 | Molecular Weight | 202.25200 | |
| Density | 1.164 g/cm3 | Boiling Point | 484.7ºC at 760 mmHg | |
| Molecular Formula | C12H14N2O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 246.9ºC | |
Use of N-AcetyltryptamineN-Acetyltryptamine is a partial agonist for melatonin receptors in the retina[1]. N-Acetyltryptamine is also used for determination of serotonin N-acetyl transferase activity[2]. |
| Name | N-acetyltryptamine |
|---|---|
| Synonym | More Synonyms |
| Description | N-Acetyltryptamine is a partial agonist for melatonin receptors in the retina[1]. N-Acetyltryptamine is also used for determination of serotonin N-acetyl transferase activity[2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.164 g/cm3 |
|---|---|
| Boiling Point | 484.7ºC at 760 mmHg |
| Molecular Formula | C12H14N2O |
| Molecular Weight | 202.25200 |
| Flash Point | 246.9ºC |
| Exact Mass | 202.11100 |
| PSA | 44.89000 |
| LogP | 2.23740 |
| Vapour Pressure | 1.51E-09mmHg at 25°C |
| Index of Refraction | 1.619 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Chloroplastic and cytoplasmic overexpression of sheep serotonin N-acetyltransferase in transgenic rice plants is associated with low melatonin production despite high enzyme activity.
J. Pineal Res. 58(4) , 461-9, (2015) Serotonin N-acetyltransferase (SNAT), the penultimate enzyme in melatonin biosynthesis, catalyzes the conversion of serotonin into N-acetylserotonin. Plant SNAT is localized in chloroplasts. To test S... |
|
|
Serine residues 110 and 114 are required for agonist binding but not antagonist binding to the melatonin MT(1) receptor.
Biochem. Biophys. Res. Commun. 282(5) , 1229-36, (2001) Site-directed mutation of serine 110 (Ser(3.35)) and serine 114 (Ser(3.39)) in the human melatonin MT(1) receptor to alanine residues reduced ligand binding affinities of seven known melatonin recepto... |
|
|
[The biosynthesis of low-molecular-weight nitrogen-containing secondary metabolite-alkaloids by the resident strains of Penicillium chrysogenum and Penicillium expansum isolated on the board of the Mir space station ].
Mikrobiologiia 71(6) , 773-7, (2002) The analysis of the absorption spectra of the low-molecular-weight nitrogen-containing secondary metabolites--alkaloids--of 4 Penicillium chrysogenum strains and 6 Penicillium expansum strains isolate... |
| N-[2-(1H-indol-3-yl)ethyl]acetamide |
| Tocris-0357 |
| Chicago Sky Blue 6B |
| N-Acetyltryptamine |
| N-(2-(1H-Indol-3-yl)ethyl)acetamide |
 CAS#:61-54-1
CAS#:61-54-1 CAS#:108-24-7
CAS#:108-24-7 CAS#:75-36-5
CAS#:75-36-5 CAS#:64-19-7
CAS#:64-19-7 CAS#:343-94-2
CAS#:343-94-2 CAS#:2735-73-1
CAS#:2735-73-1 CAS#:100-63-0
CAS#:100-63-0 CAS#:63050-21-5
CAS#:63050-21-5 CAS#:542-59-6
CAS#:542-59-6![N-[2-[1,2-bis(3-methylbut-2-enyl)indol-3-yl]ethyl]acetamide structure](https://image.chemsrc.com/caspic/172/23690-26-8.png) CAS#:23690-26-8
CAS#:23690-26-8 CAS#:73-31-4
CAS#:73-31-4 CAS#:486-84-0
CAS#:486-84-0![N-[2-(1H-Indol-3-yl)ethyl]ethanethioamide structure](https://image.chemsrc.com/caspic/217/10022-74-9.png) CAS#:10022-74-9
CAS#:10022-74-9 CAS#:2731-06-8
CAS#:2731-06-8 CAS#:518-06-9
CAS#:518-06-9![2-(1H-indol-3-yl)-2'-methyl-4',5'-dihydrospiro[indoline-3,3'-pyrrole] structure](https://image.chemsrc.com/caspic/230/122709-55-1.png) CAS#:122709-55-1
CAS#:122709-55-1