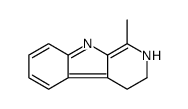
Harmane

Harmane structure
|
Common Name | Harmane | ||
|---|---|---|---|---|
| CAS Number | 486-84-0 | Molecular Weight | 182.221 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 386.9±22.0 °C at 760 mmHg | |
| Molecular Formula | C12H10N2 | Melting Point | 235-238 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 176.2±13.6 °C | |
Use of HarmaneHarmane, a β-Carboline alkaloid (BCA), is a potent neurotoxin that causes severe action tremors and psychiatric manifestations. Harmane shows 1000-fold selectivity for I1-Imidazoline receptor (IC50=30 nM) over α2-adrenoceptor (IC50=18 μM). Harmane is also a potent and selective inhibitor of monoamine oxidase (MAO) (IC50s=0.5 and 5 μM for human MAO A/B, respectively). Harmane exhibits comutagenic effect[1][2][3][4]. |
| Name | harman |
|---|---|
| Synonym | More Synonyms |
| Description | Harmane, a β-Carboline alkaloid (BCA), is a potent neurotoxin that causes severe action tremors and psychiatric manifestations. Harmane shows 1000-fold selectivity for I1-Imidazoline receptor (IC50=30 nM) over α2-adrenoceptor (IC50=18 μM). Harmane is also a potent and selective inhibitor of monoamine oxidase (MAO) (IC50s=0.5 and 5 μM for human MAO A/B, respectively). Harmane exhibits comutagenic effect[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
hMAO-A:0.5 μM (IC50) MAO-B:5 μM (IC50) α2-adrenergic receptor:18 μM (IC50) I1-Imidazoline receptor:30 nM (IC50) |
| References |
[3]. Glover V, et, al. β-Carbolines as selective monoamine oxidase inhibitors:In vivo implications |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 386.9±22.0 °C at 760 mmHg |
| Melting Point | 235-238 °C(lit.) |
| Molecular Formula | C12H10N2 |
| Molecular Weight | 182.221 |
| Flash Point | 176.2±13.6 °C |
| Exact Mass | 182.084396 |
| PSA | 28.68000 |
| LogP | 3.26 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.750 |
| InChIKey | PSFDQSOCUJVVGF-UHFFFAOYSA-N |
| SMILES | Cc1nccc2c1[nH]c1ccccc12 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | 20/21 |
| Safety Phrases | S22-S24/25 |
| RIDADR | 1544 |
| WGK Germany | 3 |
| RTECS | UV0280000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
|
|
Genetic mapping of targets mediating differential chemical phenotypes in Plasmodium falciparum.
Nat. Chem. Biol. 5 , 765-71, (2009) Studies of gene function and molecular mechanisms in Plasmodium falciparum are hampered by difficulties in characterizing and measuring phenotypic differences between individual parasites. We screened... |
|
|
The use of natural product scaffolds as leads in the search for trypanothione reductase inhibitors
Bioorg. Med. Chem. 16 , 6689, (2008) Twenty-three heterocyclic compounds were evaluated for their potential as trypanothione reductase inhibitors. As a result, the harmaline, 10-thiaisoalloxazine, and aspidospermine frameworks were ident... |
| 1-Methyl-9H-β-carboline |
| 2-Methyl-b-carboline |
| Harmane |
| Aribine |
| MFCD00004957 |
| Harman |
| 1-Methyl-β-carboline |
| 1-methylbetacarboline |
| Aribin |
| 1-Methyl-9H-pyrid[3,4-b]indole |
| EINECS 207-642-2 |
| 9H-Pyrido[3,4-b]indole, 1-methyl- |
| Passiflorin |
| Loturine |
| 1-methyl-9H-pyrido[3,4-b]indole |
| 1-Me-carboline |
| Locuturine |
| Locuturin |
| Phorbol 12,13-dibutyrate |
 CAS#:525-41-7
CAS#:525-41-7 CAS#:5159-20-6
CAS#:5159-20-6![1H-Pyrido[3,4-b]indole,2,3,4,9-tetrahydro-1-methyl- Structure](https://image.chemsrc.com/caspic/473/2506-10-7.png) CAS#:2506-10-7
CAS#:2506-10-7 CAS#:75-91-2
CAS#:75-91-2 CAS#:244-63-3
CAS#:244-63-3 CAS#:1016-47-3
CAS#:1016-47-3 CAS#:127-19-5
CAS#:127-19-5 CAS#:82980-12-9
CAS#:82980-12-9![1H-Pyrido[3,4-b]indole-3-carboxylicacid, 2,3,4,9-tetrahydro-1-methyl- Structure](https://image.chemsrc.com/caspic/209/5470-37-1.png) CAS#:5470-37-1
CAS#:5470-37-1 CAS#:15071-56-4
CAS#:15071-56-4![2,3-diethyl-12H-indolo[2,3-a]quinolizin-5-ium,bromide structure](https://image.chemsrc.com/caspic/266/139332-12-0.png) CAS#:139332-12-0
CAS#:139332-12-0![1-methyl-9H-pyrido[3,4-b]indol-6-amine structure](https://image.chemsrc.com/caspic/030/102206-91-7.png) CAS#:102206-91-7
CAS#:102206-91-7![1-methyl-8-nitro-9H-pyrido[3,4-b]indole structure](https://image.chemsrc.com/caspic/019/102207-02-3.png) CAS#:102207-02-3
CAS#:102207-02-3![1-methyl-6-nitro-9H-pyrido[3,4-b]indole structure](https://image.chemsrc.com/caspic/216/38314-91-9.png) CAS#:38314-91-9
CAS#:38314-91-9![methyl 2-(9H-pyrido[3,4-b]indol-1-yl)acetate structure](https://image.chemsrc.com/caspic/302/138428-34-9.png) CAS#:138428-34-9
CAS#:138428-34-9 CAS#:20127-63-3
CAS#:20127-63-3![9H-Pyrido[3,4-b]indole, 6-bromo-1-methyl- structure](https://image.chemsrc.com/caspic/070/18813-71-3.png) CAS#:18813-71-3
CAS#:18813-71-3 CAS#:2521-07-5
CAS#:2521-07-5
