Ciprofloxacin Hydrochloride

Ciprofloxacin Hydrochloride structure
|
Common Name | Ciprofloxacin Hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 102-92-1 | Molecular Weight | 367.802 | |
| Density | 1.199 g/cm3 | Boiling Point | 256-258 °C(lit.) | |
| Molecular Formula | C9H7ClO | Melting Point | 35-37 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | >230 °F | |
| Symbol |

GHS05 |
Signal Word | Danger | |
| Name | Cinnamoyl chloride |
|---|---|
| Synonym | More Synonyms |
| Density | 1.199 g/cm3 |
|---|---|
| Boiling Point | 256-258 °C(lit.) |
| Melting Point | 35-37 °C(lit.) |
| Molecular Formula | C9H7ClO |
| Molecular Weight | 367.802 |
| Flash Point | >230 °F |
| Exact Mass | 367.109894 |
| PSA | 17.07000 |
| LogP | 2.46520 |
| Vapour Pressure | 0.0143mmHg at 25°C |
| Index of Refraction | 1.614 (20ºC) |
| Storage condition | 0-6°C |
| Water Solubility | DECOMPOSES |
| Symbol |

GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H314 |
| Precautionary Statements | P260-P280-P303 + P361 + P353-P304 + P340 + P310-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | C:Corrosive |
| Risk Phrases | R34 |
| Safety Phrases | S26-S36/37/39-S45-S25 |
| RIDADR | UN 3261 8/PG 2 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 8 |
| HS Code | 2916399090 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2916399090 |
|---|---|
| Summary | 2916399090 other aromatic monocarboxylic acids, their anhydrides, halides, peroxides, peroxyacids and their derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Light- and temperature-responsive liposomes incorporating cinnamoyl Pluronic F127.
Int. J. Pharm. 468(1-2) , 243-9, (2014) Light- and temperature-responsive liposomes were prepared by immobilizing cinnamoyl Pluronic F127 (CP F127) on the surface of egg phosphatidylcholine liposomes. CP F127 was prepared by a condensation ... |
|
|
[A synthesis of cinnamoyloxyisoflavones].
Bioorg. Khim. 32(4) , 446-7, (2006) Interaction of 7-hydroxyisoflavonones with cinnamoyl chloride results in cinnamoyloxyisoflavonones. |
|
|
Synthesis and antitumor activity of lapathoside D and its analogs
Eur. J. Med. Chem. 53 , 1-12, (2012) Phenylpropanoid sucrose esters are important class of plant-derived natural products and have greater potential to be leads for new drugs because of their structural diversity and broad-array of pharm... |
| MFCD00000732 |
| 1-Cyclopropyl-6-fluoro-4-oxo-7-(1-piperazinyl)-1,4-dihydro-3-quinolinecarboxylic acid hydrochloride (1:1) |
| 2-PROPENOYL CHLORIDE,3-PHENYL- |
| Cinnamoyl Chloride |
| CINNAMIC ACID CHLORIDE |
| 1-Cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic acid hydrochloride (1:1) |
| Ciamoyl chloride |
| 3-PHENYL-2-PROPENOYL CHLORIDE |
| CINNAMONCHLORIDE |
| Cinnamic chloride |
| Phenylacrylchloride |
| 4-(3-carboxy-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydroquinolin-7-yl)piperazin-1-ium chloride |
| ciprofloxacin monohydrochloride |
| Ciprofloxacin Hcl |
| 1-Cyclopropyl-6-fluor-4-oxo-7-piperazin-1-yl-1,4-dihydrochinolin-3-carbonsäurehydrochlorid |
| 3-Quinolinecarboxylic acid, 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-, hydrochloride (1:1) |
| Cinnamoyl |
| Phenylacrylyl chloride |
| PhCH=CHCOCl |
| Cinnamoylchloride |
| acide 1-cyclopropyl-6-fluoro-4-oxo-7-pipérazin-1-yl-1,4-dihydroquinoléine-3-carboxylique chlorhydrate |
| CINNAMOYL CHLOCIDE |
| 1-cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-dihydroquinoline-3-carboxylic acid hydrochloride |
| (2E)-3-phenylprop-2-enoyl chloride |
| Ciprofloxacin Hydrochloride |
| EINECS 203-065-5 |
 CAS#:140-10-3
CAS#:140-10-3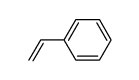 CAS#:292638-84-7
CAS#:292638-84-7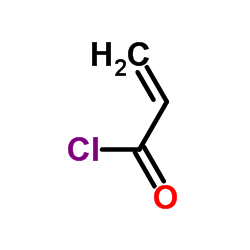 CAS#:814-68-6
CAS#:814-68-6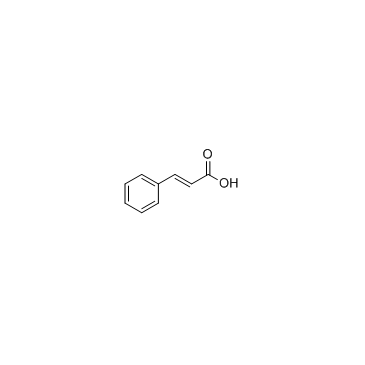 CAS#:621-82-9
CAS#:621-82-9 CAS#:100-52-7
CAS#:100-52-7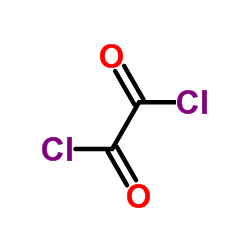 CAS#:79-37-8
CAS#:79-37-8 CAS#:50966-31-9
CAS#:50966-31-9 CAS#:105703-70-6
CAS#:105703-70-6 CAS#:109853-54-5
CAS#:109853-54-5 CAS#:10579-65-4
CAS#:10579-65-4 CAS#:111917-09-0
CAS#:111917-09-0 CAS#:606-86-0
CAS#:606-86-0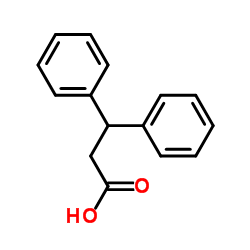 CAS#:606-83-7
CAS#:606-83-7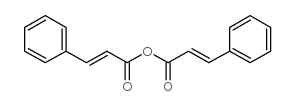 CAS#:538-56-7
CAS#:538-56-7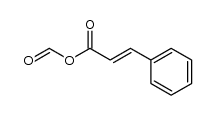 CAS#:103884-33-9
CAS#:103884-33-9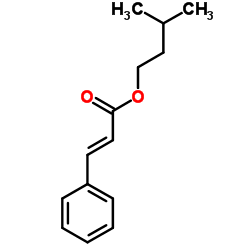 CAS#:3487-99-8
CAS#:3487-99-8 CAS#:491-67-8
CAS#:491-67-8
