ITI214 free base
Modify Date: 2025-08-24 23:10:14
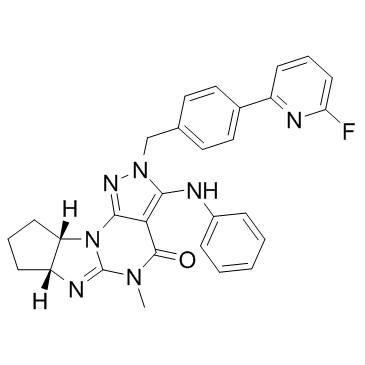
ITI214 free base structure
|
Common Name | ITI214 free base | ||
|---|---|---|---|---|
| CAS Number | 1160521-50-5 | Molecular Weight | 507.561 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 731.7±70.0 °C at 760 mmHg | |
| Molecular Formula | C29H26FN7O | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 396.3±35.7 °C | |
Use of ITI214 free baseITI-214 (free base) is a picomolar PDE1 inhibitor with excellent selectivity against other PDE family members and against a panel of enzymes, receptors, transporters, and ion channels, exhibits potent PDE1 inhibitory activity (Ki = 58 pM).IC50 value: 58 pM (Ki)Target: PDE1in vitro: ITI-214 exhibits picomolar inhibitory potency for PDE1, demonstrates excellent selectivity against all other PDE families. ITI214 exhibits excellent selectivity over other PDE familymembers. For instance, the Ki values of ITI214 against recombinant full-length human PDE1A, PDE1B, and PDE1C are 33 pM, 380 pM, and 35 pM, respectively. ITI214 is profiled in a panel of enzymes, receptors, transporters, and ion channels from Caliper at 10 μM, which is over 170000 times higher than its Ki for PDE1, and demonstrates good selectivity. [1]in vivo: ITI214 possesses a good overall profile with balanced physicochemical properties, excellent potency and selectivity, and good pharmacokinetics. ITI214 is found to significantly enhance memory performance in the test with a minimum effective dose of 3 mg/kg. [1] |
| Name | ITI214 free base |
|---|---|
| Synonym | More Synonyms |
| Description | ITI-214 (free base) is a picomolar PDE1 inhibitor with excellent selectivity against other PDE family members and against a panel of enzymes, receptors, transporters, and ion channels, exhibits potent PDE1 inhibitory activity (Ki = 58 pM).IC50 value: 58 pM (Ki)Target: PDE1in vitro: ITI-214 exhibits picomolar inhibitory potency for PDE1, demonstrates excellent selectivity against all other PDE families. ITI214 exhibits excellent selectivity over other PDE familymembers. For instance, the Ki values of ITI214 against recombinant full-length human PDE1A, PDE1B, and PDE1C are 33 pM, 380 pM, and 35 pM, respectively. ITI214 is profiled in a panel of enzymes, receptors, transporters, and ion channels from Caliper at 10 μM, which is over 170000 times higher than its Ki for PDE1, and demonstrates good selectivity. [1]in vivo: ITI214 possesses a good overall profile with balanced physicochemical properties, excellent potency and selectivity, and good pharmacokinetics. ITI214 is found to significantly enhance memory performance in the test with a minimum effective dose of 3 mg/kg. [1] |
|---|---|
| Related Catalog | |
| References |
[2]. Lawrence Wennogle. Novel uses. From PCT Int. Appl. (2014), WO 2014145617 A2 20140918. [3]. Peng Li , et al. Salt crystals. From PCT Int. Appl. (2013), WO 2013192556 A2 20131227. [4]. Allen A. Fienberg, et al. Organic compounds. From PCT Int. Appl. (2010), WO 2010132127 A1 20101118. |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 731.7±70.0 °C at 760 mmHg |
| Molecular Formula | C29H26FN7O |
| Molecular Weight | 507.561 |
| Flash Point | 396.3±35.7 °C |
| Exact Mass | 507.218292 |
| LogP | 2.57 |
| Vapour Pressure | 0.0±2.4 mmHg at 25°C |
| Index of Refraction | 1.754 |
| (11R,15S)-4-{[4-(6-fluoropyridin-2-yl)phenyl]methyl}-8-methyl-5-(phenylamino)-1,3,4,8,10-pentaazatetracyclo[7.6.0.02,6.011,15]pentadeca-2,5,9-trien-7-one |
| Cyclopent[4,5]imidazo[1,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one, 2-[[4-(6-fluoro-2-pyridinyl)phenyl]methyl]-5,6a,7,8,9,9a-hexahydro-5-methyl-3-(phenylamino)-, (6aR,9aS)- |
| (6aR,9aS)-3-Anilino-2-[4-(6-fluoro-2-pyridinyl)benzyl]-5-methyl-5,6a,7,8,9,9a-hexahydrocyclopenta[4,5]imidazo[1,2-a]pyrazolo[4,3-e]pyrimidin-4(2H)-one |
| ITI214 (free base) |