AFLATOXIN B1
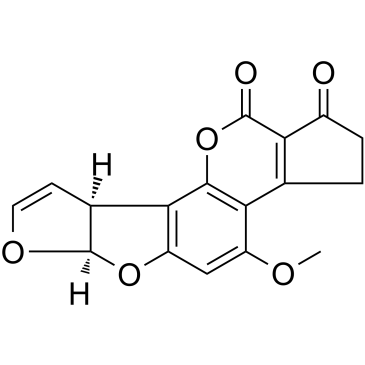
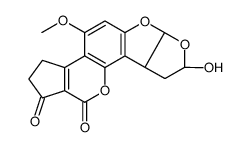
AFLATOXIN B1 structure
|
Common Name | AFLATOXIN B1 | ||
|---|---|---|---|---|
| CAS Number | 1162-65-8 | Molecular Weight | 312.274 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 528.2±50.0 °C at 760 mmHg | |
| Molecular Formula | C17H12O6 | Melting Point | 268-269 °C | |
| MSDS | Chinese USA | Flash Point | 237.7±30.2 °C | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
| Purity | Quantity | Budget | Inquiry |
|---|
Use of AFLATOXIN B1Aflatoxin B1 (AFB1) is a Class 1A carcinogen, which is a secondary metabolite of Aspergillus flavus and A. parasiticus. Aflatoxin B1 (AFB1) mainly induces the transversion of G-->T in the third position of codon 249 of the p53 tumor suppressor gene, resulting in mutation[1][2]. |
| Name | aflatoxin B1 |
|---|---|
| Synonym | More Synonyms |
| Description | Aflatoxin B1 (AFB1) is a Class 1A carcinogen, which is a secondary metabolite of Aspergillus flavus and A. parasiticus. Aflatoxin B1 (AFB1) mainly induces the transversion of G-->T in the third position of codon 249 of the p53 tumor suppressor gene, resulting in mutation[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 528.2±50.0 °C at 760 mmHg |
| Melting Point | 268-269 °C |
| Molecular Formula | C17H12O6 |
| Molecular Weight | 312.274 |
| Flash Point | 237.7±30.2 °C |
| Exact Mass | 312.063385 |
| PSA | 74.97000 |
| LogP | 0.45 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.687 |
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300 + H310 + H330-H350 |
| Precautionary Statements | P201-P260-P264-P280-P284-P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T+,T,Xn,F |
| Risk Phrases | 45-46-26/27/28-36-20/21/22-11-65-48/23/24/25-36/38-39/23/24/25-23/24/25 |
| Safety Phrases | 53-45-36-26-16-24-7 |
| RIDADR | UN 3462 6.1/PG 1 |
| WGK Germany | 3 |
| RTECS | GY1925000 |
| Packaging Group | I |
|
~24% 
AFLATOXIN B1 CAS#:1162-65-8 |
| Literature: Trost, Barry M.; Toste, F. Dean Journal of the American Chemical Society, 2003 , vol. 125, # 10 p. 3090 - 3100 |
|
~% 
AFLATOXIN B1 CAS#:1162-65-8 |
| Literature: Journal of the American Chemical Society, , vol. 120, # 25 p. 6231 - 6239 |
|
~% 
AFLATOXIN B1 CAS#:1162-65-8 |
| Literature: Journal of Organic Chemistry, , vol. 59, # 16 p. 4424 - 4429 |
|
~% 
AFLATOXIN B1 CAS#:1162-65-8 |
| Literature: Journal of Organic Chemistry, , vol. 59, # 16 p. 4424 - 4429 |
|
Low toxicity of deoxynivalenol-3-glucoside in microbial cells.
Toxins (Basel.) 7(1) , 187-200, (2015) Host plants excrete a glucosylation enzyme onto the plant surface that changes mycotoxins derived from fungal secondary metabolites to glucosylated products. Deoxynivalenol-3-glucoside (DON3G) is synt... |
|
|
Aflatoxins contamination in Pakistani brown rice: a comparison of TLC, HPLC, LC-MS/MS and ELISA techniques.
Toxicol. Mech. Methods 24(8) , 544-51, (2014) Advancement in the field of analytical food-chemistry has explored various experimental techniques for aflatoxins (AFs) quantification. The present study was aimed to compare four different techniques... |
|
|
Deoxynivalenol-mimic nanobody isolated from a naïve phage display nanobody library and its application in immunoassay.
Anal. Chim. Acta 887 , 201-8, (2015) In this study, using mycotoxin deoxynivalenol (DON) as a model hapten, we developed a nanobody-based environmental friendly immunoassay for sensitive detection of DON. Two nanobodies (N-28 and N-31) w... |
| Cyclopenta[c]furo[3',2':4,5]furo[2,3-h][1]benzopyran-1,11-dione, 2,3,6a,9a-tetrahydro-4-methoxy-, (6aR,9aS)- |
| Aflatoxin B1 |
| Aflatoxin B1 Crystalline |
| (-)-Aflatoxin B1 |
| (6aR,9aS)-4-Methoxy-2,3,6a,9a-tetrahydrocyclopenta[c]furo[3',2':4,5]furo[2,3-h]chromene-1,11-dione |
| Aflatoxin B1, crystalline |
| MFCD00869647 |
| Aflatoxin |
| EINECS 214-603-3 |