Dantron
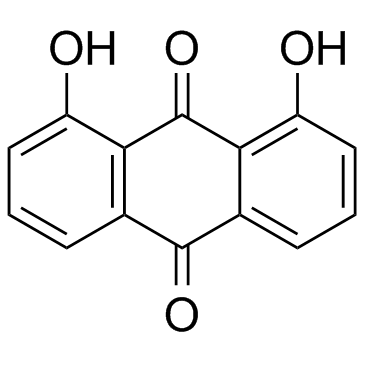
Dantron structure
|
Common Name | Dantron | ||
|---|---|---|---|---|
| CAS Number | 117-10-2 | Molecular Weight | 240.211 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 452.7±35.0 °C at 760 mmHg | |
| Molecular Formula | C14H8O4 | Melting Point | 191-193 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 241.7±22.4 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Warning | |
Use of DantronDanthron is a natural product extracted from the traditional Chinese medicine rhubarb. Danthron functions in regulating glucose and lipid metabolism by activating AMPK. |
| Name | chrysazin |
|---|---|
| Synonym | More Synonyms |
| Description | Danthron is a natural product extracted from the traditional Chinese medicine rhubarb. Danthron functions in regulating glucose and lipid metabolism by activating AMPK. |
|---|---|
| Related Catalog | |
| Target |
AMPK |
| In Vitro | Danthron (0.1, 1, and 10 μM) dose-dependently promotes the phosphorylation of AMPK and acetyl-CoA carboxylase (ACC) in both HepG2 and C2C12 cells. Meanwhile, Danthron treatment significantly reduces the lipid synthesis related sterol regulatory element-binding protein 1c (SREBP1c) and fatty acid synthetase (FAS) gene expressions, and the total cholesterol (TC) and triglyceride (TG) levels. In addition, Danthron treatment efficiently increases glucose consumption. Danthron effectively reduces intracellular lipid contents and enhances glucose consumption in vitro via activation of AMPK signaling pathway. 10 μM Danthron/24 h is safe for HepG2 cells. With 80% confluence, HepG2 cells are incubated with Danthron (0.1-10 μM) in FBS-Free media for 8 h. Subsequently, cells are harvested for Western blot assay. Danthron increases the p-AMPK protein in a dose-dependent manner, and no changes in t-AMPK protein are observed[1]. Danthron inhibits 9-cis retinoic acid (9cRA)-induced retinoic X receptor α (RXRα) transactivation by IC50 at 0.11 μM. To further clarify the stoichimetric ratio of Danthron binding to RXRα-ligand-binding domain (LBD), isothermal titration calorimetry (ITC) experiment is performed. The KD value of Danthron binds to RXRα-LBD by ITC experiment is determined at 7.5 μM[2]. |
| In Vivo | Danthron functions as an insulin sensitizer in vivo. Danthron improves insulin sensitivity in diet-induced obese (DIO) mice. The insulin tolerance test result shows that Danthron (5 mg/kg) treated diet-induced obesity mice exhibit lower glucose levels after insulin challenge, compared with the control vehicle-treated group[2]. |
| Cell Assay | HepG2 cells are transfected with pGL3-ABCA1 promoter-luc or pGL3-ABCG1 promoter-luc and pRL-SV40 plasmids. At 6 h post-transfection, the cells are incubated with Danthron (0-20 μM), TO90 (2 μM) or DMSO for 24 h. Luciferase activity is measured using the Dual Luciferase Reporter Assay kit[1]. |
| Animal Admin | Mice[2] C57/BL6 male mice are fed with a high fat diet for 3 months and treated with Danthron (5 mg/kg) or vehicle orally for 8 weeks. The animals are then fasted for 6 h and then given intraperitoneal injection of insulin at 1.5 units/kg. Blood samples are analyzed at 15, 30, 45, 60, 90, and 120 min using Accu-Chek active blood sugar test meter. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 452.7±35.0 °C at 760 mmHg |
| Melting Point | 191-193 °C(lit.) |
| Molecular Formula | C14H8O4 |
| Molecular Weight | 240.211 |
| Flash Point | 241.7±22.4 °C |
| Exact Mass | 240.042252 |
| PSA | 74.60000 |
| LogP | 4.57 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.733 |
| Storage condition | Refrigerator |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H319-H351 |
| Precautionary Statements | P281-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R40 |
| Safety Phrases | S36/37 |
| RIDADR | 2811 |
| WGK Germany | 1 |
| RTECS | CB6650000 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 29146990 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914690090 |
|---|---|
| Summary | 2914690090 other quinones。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
|
Safety assessment of dietary bamboo charcoal powder: a 90-day subchronic oral toxicity and mutagenicity studies.
Food Chem. Toxicol. 75 , 50-7, (2015) Vegetable carbon has been used as food additive in EU (E153) and China for many years; however, no experimental data have been available on its dietary safety. This study was designed to evaluate the ... |
|
|
Determination of hydroxylated polycyclic aromatic hydrocarbons by HPLC-photoionization tandem mass spectrometry in wood smoke particles and soil samples.
Anal. Bioanal. Chem 407 , 4523-34, (2015) A simple and fast method for analysis of hydroxylated polycyclic aromatic hydrocarbons using pressurized liquid extraction and high performance liquid chromatography utilizing photoionization tandem m... |
|
|
1,4-dihydroxyanthraquinone electrochemical sensor based on molecularly imprinted polymer using multi-walled carbon nanotubes and multivariate optimization method.
Talanta 146 , 525-32, (2015) The present work describes development of a simple and cost-effective electrochemical sensor for determination of 1,4-dihydroxyanthraquinone (1,4-DAQ) based on molecularly imprinted polypyrrole (PPY).... |
| EINECS 204-173-5 |
| ALTAN |
| Chrysazin |
| Dionone |
| Modane |
| Laxapur |
| roydan |
| prugol |
| Duolax |
| Danivac |
| 1,8-dihydroxy-anthraquinone |
| Istin |
| LTAN |
| Dorbane |
| Dantron |
| 1,8-Dihydroxy-9,10-anthraquinone |
| 1,8-Dihydroxyanthraquinone |
| 9,10-Anthracenedione, 1,8-dihydroxy- |
| MFCD00001211 |
| Danthron |
 CAS#:577975-52-1
CAS#:577975-52-1![3-(methoxymethoxy)-2-(4-oxo-4,5,6,7-tetrahydrobenzo[d]isoxazol-3-yl)benzaldehyde Structure](https://image.chemsrc.com/caspic/220/577975-37-2.png) CAS#:577975-37-2
CAS#:577975-37-2![5a-hydroxy-10-(methoxymethoxy)-3,4,5,5a-tetrahydro-6H-anthra[9,1-cd]isoxazol-6-one Structure](https://image.chemsrc.com/caspic/144/577975-39-4.png) CAS#:577975-39-4
CAS#:577975-39-4![3-(2-(1,3-dioxan-2-yl)-6-(methoxymethoxy)phenyl)-6,7-dihydrobenzo[d]isoxazol-4(5H)-one Structure](https://image.chemsrc.com/caspic/458/577975-53-2.png) CAS#:577975-53-2
CAS#:577975-53-2 CAS#:1963-82-2
CAS#:1963-82-2 CAS#:69043-83-0
CAS#:69043-83-0 CAS#:89646-25-3
CAS#:89646-25-3 CAS#:89646-23-1
CAS#:89646-23-1 CAS#:129-39-5
CAS#:129-39-5 CAS#:480-22-8
CAS#:480-22-8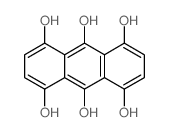 CAS#:23478-60-6
CAS#:23478-60-6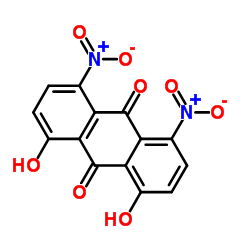 CAS#:81-55-0
CAS#:81-55-0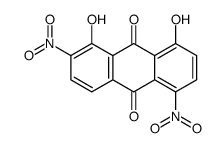 CAS#:39003-36-6
CAS#:39003-36-6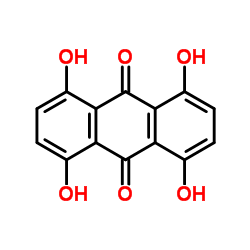 CAS#:81-60-7
CAS#:81-60-7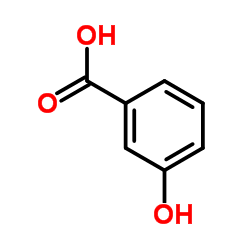 CAS#:99-06-9
CAS#:99-06-9 CAS#:69-72-7
CAS#:69-72-7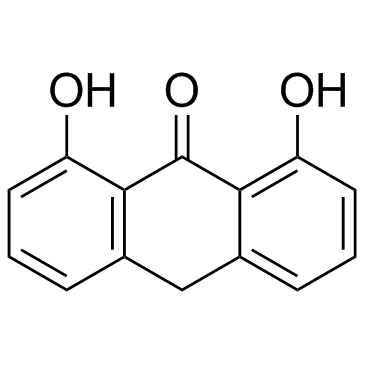 CAS#:1143-38-0
CAS#:1143-38-0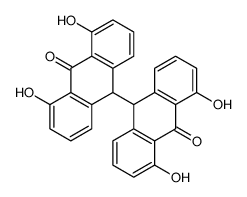 CAS#:31991-54-5
CAS#:31991-54-5
