Boc-Leu-OH.H2O
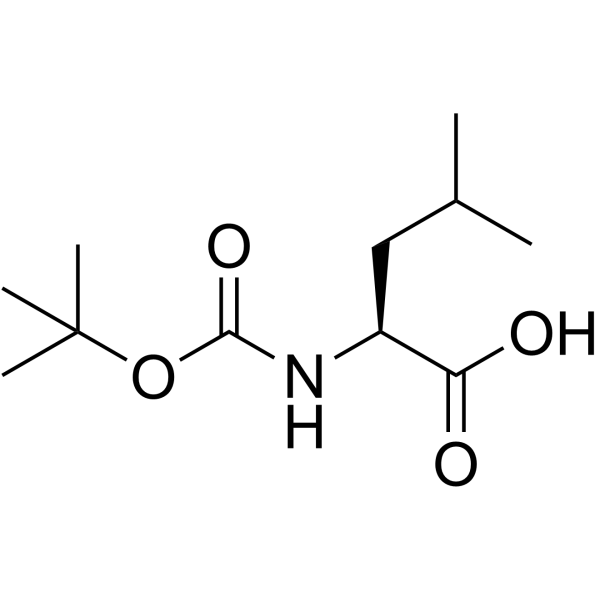
Boc-Leu-OH.H2O structure
|
Common Name | Boc-Leu-OH.H2O | ||
|---|---|---|---|---|
| CAS Number | 13139-15-6 | Molecular Weight | 231.289 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 339.7±44.0 °C at 760 mmHg | |
| Molecular Formula | C11H21NO4 | Melting Point | 85-87 °C | |
| MSDS | Chinese USA | Flash Point | 159.2±28.4 °C | |
Use of Boc-Leu-OH.H2OBoc-L-Leu-OH is a leucine derivative[1]. |
| Name | N(α)-t-butoxycarbonyl-L-leucine |
|---|---|
| Synonym | More Synonyms |
| Description | Boc-L-Leu-OH is a leucine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 339.7±44.0 °C at 760 mmHg |
| Melting Point | 85-87 °C |
| Molecular Formula | C11H21NO4 |
| Molecular Weight | 231.289 |
| Flash Point | 159.2±28.4 °C |
| Exact Mass | 231.147064 |
| PSA | 75.63000 |
| LogP | 2.99 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.472 |
| InChIKey | MDXGYYOJGPFFJL-QMMMGPOBSA-N |
| SMILES | CC(C)CC(NC(=O)OC(C)(C)C)C(=O)O |
| Storage condition | −20°C |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Transport and signaling via the amino acid binding site of the yeast Gap1 amino acid transceptor.
Nat. Chem. Biol. 5 , 45-52, (2009) Transporter-related nutrient sensors, called transceptors, mediate nutrient activation of signaling pathways through the plasma membrane. The mechanism of action of transporting and nontransporting tr... |
|
|
In situ fabrication of cleavable peptide arrays on polydimethylsiloxane and applications for kinase activity assays.
Anal. Chim. Acta 865 , 53-9, (2015) Polydimethylsiloxane (PDMS) is widely used for microfabrication and bioanalysis; however, its surface functionalization is limited due to the lack of active functional groups and incompatibility with ... |
|
|
Concise synthesis of PM-94128 and Y-05460M-A.
J. Org. Chem. 74(19) , 7566-9, (2009) The enantioselective total synthesis of PM-94128, a potent cytotoxin of microbial origin, was accomplished by a concise nine-step sequence of reactions in 14% overall yield from N-Boc-l-leucine. The s... |
| N-Boc-L-leucine hydrate |
| (E)-N-{Hydroxy[(2-methyl-2-propanyl)oxy]methylene}-L-leucine |
| (S)-2-((tert-Butoxycarbonyl)amino)-4-methylpentanoic acid |
| MFCD00066067 |
| L-LEUCINE-N-T-BOC:H2O |
| L-Leucine, N-[(1,1-dimethylethoxy)carbonyl]- |
| BOC-LEU |
| boc-leu-oh.h20 |
| EINECS 236-073-2 |
| N-tert-butoxycarbonyl-L-leucine |
| BOC-L-LEUCINE-OH |
| N-{[(2-Methyl-2-propanyl)oxy]carbonyl}-L-leucine |
| N-tert-butyloxycarbonyl-L-leucine |
| BOC-L-Leucine |
| N(alpha)-t-butoxycarbonyl-L-leucine |
| Boc-Leu-OH,H2O |
| Boc-Leu-OH.H2O |
| N-Boc-L-leucine Monohydrate |
| L-Leucine, N-[(1,1-dimethylethoxy)hydroxymethylene]-, (E)- |
| BOC-L-LEU-OH |
| BOC-LEU-OH |
| N-(tert-Butoxycarbonyl)-L-leucine Monohydrate |
| Boc-Leu-OH Monohydrate |
| N-Boc-L-leucine |
 CAS#:61-90-5
CAS#:61-90-5 CAS#:24424-99-5
CAS#:24424-99-5 CAS#:82010-31-9
CAS#:82010-31-9 CAS#:98015-52-2
CAS#:98015-52-2 CAS#:77163-64-5
CAS#:77163-64-5 CAS#:2033-24-1
CAS#:2033-24-1 CAS#:57933-83-2
CAS#:57933-83-2![L-Leucine, N-[(1,1-dimethylethoxy)carbonyl]-, 2-oxo-2-phenylethyl ester Structure](https://image.chemsrc.com/caspic/313/91287-28-4.png) CAS#:91287-28-4
CAS#:91287-28-4 CAS#:4399-40-0
CAS#:4399-40-0 CAS#:53363-89-6
CAS#:53363-89-6 CAS#:59880-97-6
CAS#:59880-97-6 CAS#:33507-63-0
CAS#:33507-63-0![[Nle11]-Substance P structure](https://image.chemsrc.com/caspic/019/57462-42-7.png) CAS#:57462-42-7
CAS#:57462-42-7 CAS#:3350-19-4
CAS#:3350-19-4![N-[(1,1-Dimethylethoxy)carbonyl]-L-leucylglycine structure](https://image.chemsrc.com/caspic/362/32991-17-6.png) CAS#:32991-17-6
CAS#:32991-17-6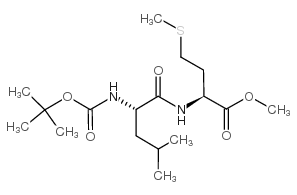 CAS#:2280-69-5
CAS#:2280-69-5 CAS#:58822-25-6
CAS#:58822-25-6
