LMK 235
Modify Date: 2024-01-02 13:32:05
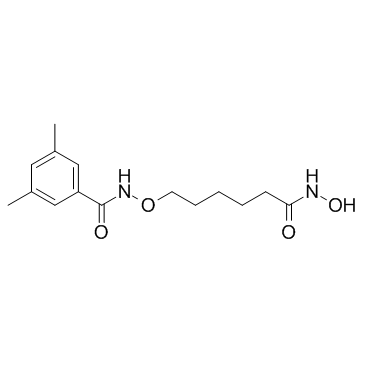
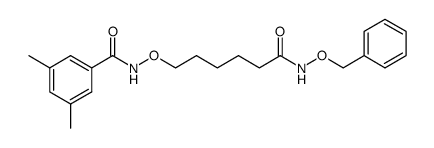
LMK 235 structure
|
Common Name | LMK 235 | ||
|---|---|---|---|---|
| CAS Number | 1418033-25-6 | Molecular Weight | 294.346 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C15H22N2O4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of LMK 235LMK-235 is a potent and selective HDAC4/5 inhibitor, inhibits HDAC5, HDAC4, HDAC6, HDAC1, HDAC2, HDAC11 and HDAC8, with IC50s of 4.22 nM, 11.9 nM, 55.7 nM, 320 nM, 881 nM, 852 nM and 1278 nM, respectively, and is used in cancer research. |
| Name | N-{[6-(Hydroxyamino)-6-oxohexyl]oxy}-3,5-dimethylbenzamide |
|---|---|
| Synonym | More Synonyms |
| Description | LMK-235 is a potent and selective HDAC4/5 inhibitor, inhibits HDAC5, HDAC4, HDAC6, HDAC1, HDAC2, HDAC11 and HDAC8, with IC50s of 4.22 nM, 11.9 nM, 55.7 nM, 320 nM, 881 nM, 852 nM and 1278 nM, respectively, and is used in cancer research. |
|---|---|
| Related Catalog | |
| Target |
HDAC5:4.22 nM (IC50) HDAC4:11.9 nM (IC50) HDAC6:55.7 nM (IC50) HDAC1:320 nM (IC50) HDAC11:852 nM (IC50) HDAC2:881 nM (IC50) HDAC8:1278 nM (IC50) |
| In Vitro | LMK-235 shows cytotoxic activity against human ovarian cancer cell lines A2780 and A2780 CisR, with IC50s of 0.49 μM and 0.32 μM, respectively. LMK-235 inhibits HDAC in A2780 and A2780 CisR cell lines, with IC50s of 0.65 μM and 0.32 μM, respectively. LMK-235 produces a higher reduction in cell viability in comparison to the combination of cisplatin and vorinostat in all cell lines[1]. LMK-235 (0, 0.625, 1.25, 2.5, 5, 10, and 20 μM) reduces the proliferation of BC cells in a dose- and time-dependent manner. LMK-235 (0-800 nM) also inhibits the growth of BC cells. Moreover, LMK-235 synergizes with bortezomib in BC cell lines[2]. LMK235 (2, 20 nM) decreases in HDAC4 nuclear accumulation in Cdkl5 -/Y NPCs, completely restores the reduced number of neurons generated from Cdkl5 -/Y NPCs. LMK235 also restores histone 3 acetylation in Cdkl5 -/Y NPCs. LMK235 causes a notable increase in the isoform IV, but does not affect BDNF isoforms I or II[3]. |
| In Vivo | LMK235 (5 and 20 mg/kg) restores survival and maturation of postmitotic granule neurons in Cdkl5 -/Y mice. LMK235 also restores synapse development in the dentate gyrus and hippocampus of Cdkl5 -/Y mice. Furthermore, LMK235 restores hippocampus-dependent learning and memory in Cdkl5 -/Y mice[3]. |
| Cell Assay | The rate of cell survival under the action of test substances is evaluated by an improved MTT assay. The assay is based on the ability of viable cells to metabolize yellow 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to violet formazan that can be detected spectrophotometrically. In brief, A2780, Cal27, Kyse510, and MDA-MB-231 cell lines are seeded at a density of 5000, 7000, 8000, and 10 000 cells/well in 96-well plates. After 24 h, cells are exposed to increased concentrations of the test compounds. Incubation is ended after 72 h, and cell survival is determined by addition of MTT solution (5 mg/mL in phosphate buffered saline). The formazan precipitate is dissolved in DMSO. Absorbance is measured at 544 and 690 nm in a FLUOstar microplate reader[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Molecular Formula | C15H22N2O4 |
| Molecular Weight | 294.346 |
| Exact Mass | 294.157959 |
| PSA | 94.64000 |
| LogP | 2.05 |
| Appearance of Characters | white to beige |
| Index of Refraction | 1.538 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: soluble20mg/mL, clear |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| N-[[6-(Hydroxyamino)-6-oxohexyl]oxy]-3,5-dimethyl-benzamide |
| Benzamide, N-[[6-(hydroxyamino)-6-oxohexyl]oxy]-3,5-dimethyl- |
| N-{[6-(Hydroxyamino)-6-oxohexyl]oxy}-3,5-dimethylbenzamide |
| LMK-235 |