tans-4-Hydroxy-D-proline hydrochloride
Modify Date: 2025-08-21 08:58:06
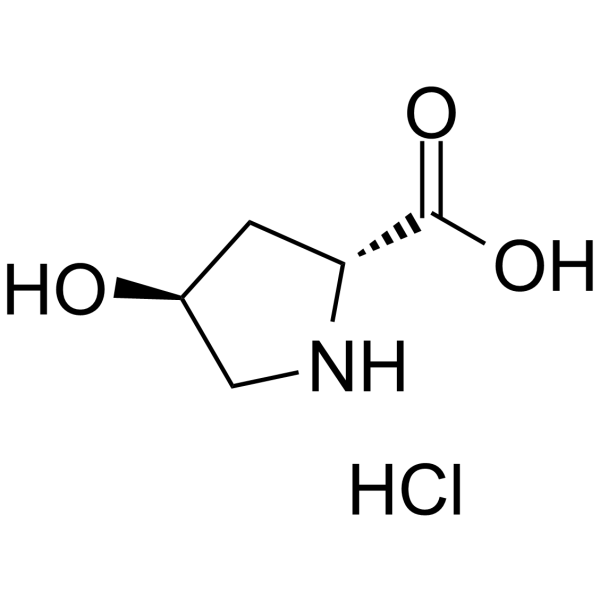
tans-4-Hydroxy-D-proline hydrochloride structure
|
Common Name | tans-4-Hydroxy-D-proline hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 142347-81-7 | Molecular Weight | 167.591 | |
| Density | N/A | Boiling Point | 368.6ºC at 760mmHg | |
| Molecular Formula | C5H10ClNO3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 176.7ºC | |
Use of tans-4-Hydroxy-D-proline hydrochloridetans-4-Hydroxy-D-proline hydrochloride is a non-cleavable ADC linker used in the synthesis of antibody-drug conjugates (ADCs). tans-4-Hydroxy-D-proline hydrochloride is also a alkyl chain-based PROTAC linker that can be used in the synthesis of PR |
| Name | trans-4-Hydroxy-D-proline hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | tans-4-Hydroxy-D-proline hydrochloride is a non-cleavable ADC linker used in the synthesis of antibody-drug conjugates (ADCs). tans-4-Hydroxy-D-proline hydrochloride is also a alkyl chain-based PROTAC linker that can be used in the synthesis of PR |
|---|---|
| Related Catalog | |
| Target |
Non-cleavable |
| In Vitro | ADCs are comprised of an antibody to which is attached an ADC cytotoxin through an ADC linker[1]. PROTACs contain two different ligands connected by a linker; one is a ligand for an E3 ubiquitin ligase and the other is for the target protein. PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins[2]. |
| References |
| Boiling Point | 368.6ºC at 760mmHg |
|---|---|
| Molecular Formula | C5H10ClNO3 |
| Molecular Weight | 167.591 |
| Flash Point | 176.7ºC |
| Exact Mass | 167.034927 |
| PSA | 69.56000 |
| Vapour Pressure | 6.22E-07mmHg at 25°C |
| InChIKey | YEJFFQAGTXBSTI-RFKZQXLXSA-N |
| SMILES | Cl.O=C(O)C1CC(O)CN1 |
| Hazard Codes | Xi |
|---|
|
~69% 
tans-4-Hydroxy-... CAS#:142347-81-7 |
| Literature: Pandey, Anil K.; Naduthambi, Devan; Thomas, Krista M.; Zondlo, Neal J. Journal of the American Chemical Society, 2013 , vol. 135, # 11 p. 4333 - 4363 |
| Precursor 1 | |
|---|---|
| DownStream 2 | |
| (2R,4S)-4-Hydroxypyrrolidine-2-carboxylic acid hydrochloride |
| D-Proline, 1-hydroxy-, hydrochloride (1:1) |
| trans-4-Hydroxy-D-proline Hydrochloride |
| 1-Hydroxy-D-proline hydrochloride (1:1) |
| (2R,4S)-4-hydroxypyrrolidine-2-carboxylic acid,hydrochloride |
| trans-4-Hydroxypyrrolidine-2-carboxylic acid hydrochloride |
| (4S)-4-Hydroxy-D-proline hydrochloride (1:1) |
| D-Proline, 4-hydroxy-, (4S)-, hydrochloride (1:1) |
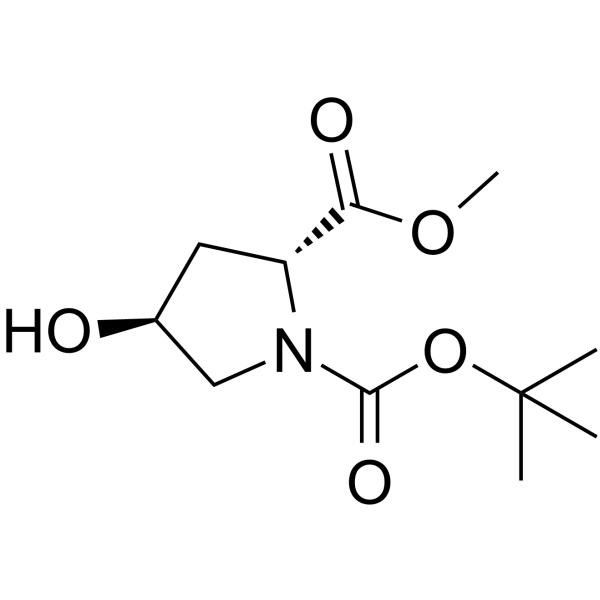
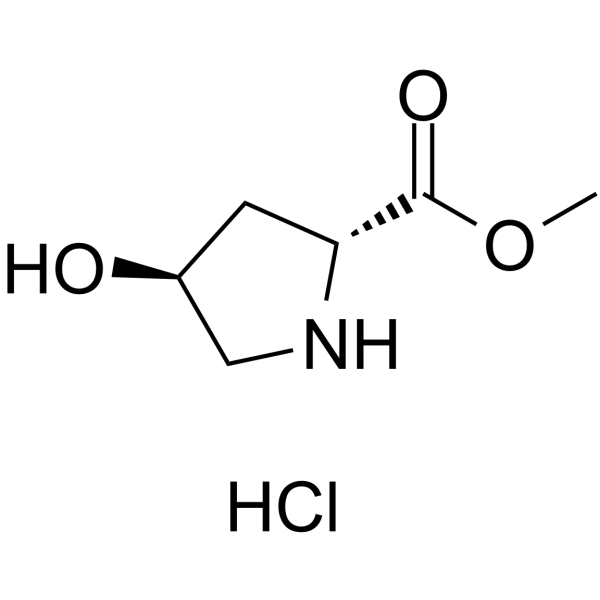 CAS#:481704-21-6
CAS#:481704-21-6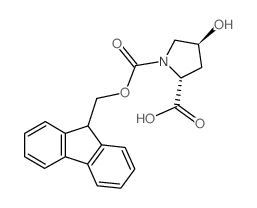 CAS#:139262-20-7
CAS#:139262-20-7
