Acetylphthalimide
Modify Date: 2025-08-21 13:11:24
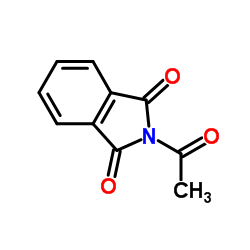
Acetylphthalimide structure
|
Common Name | Acetylphthalimide | ||
|---|---|---|---|---|
| CAS Number | 1971-49-9 | Molecular Weight | 189.167 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 345.4±25.0 °C at 760 mmHg | |
| Molecular Formula | C10H7NO3 | Melting Point | 139 °C(lit.) | |
| MSDS | USA | Flash Point | 165.8±15.5 °C | |
| Name | 2-acetylisoindole-1,3-dione |
|---|---|
| Synonym | More Synonyms |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 345.4±25.0 °C at 760 mmHg |
| Melting Point | 139 °C(lit.) |
| Molecular Formula | C10H7NO3 |
| Molecular Weight | 189.167 |
| Flash Point | 165.8±15.5 °C |
| Exact Mass | 189.042587 |
| PSA | 54.45000 |
| LogP | 0.17 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.617 |
| Safety Phrases | S22-S24/25 |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| Precursor 9 | |
|---|---|
| DownStream 7 | |
|
The hydrolysis and cyclization of some phthalamic acid derivatives.
J. Am. Chem. Soc. 88(19) , 4468-74, (1966)
|
|
|
Rhodium-Catalyzed Synthesis and Reactions of N-Acylphthalimides. Li G, et al.
Asian J. Org. Chem. 2(11) , 983-88, (2013)
|
|
|
Structure and Synthesis of the Major Components in the Hairpencil Secretion of a Male Butterfly, Lycorea ceres ceres (Cramer) 1. Meinwald J and Meinwald YC.
J. Am. Chem. Soc. 88(6) , 1305-1310, (1966)
|
| N-Acetylphthalimide |
| 2-Acetylisoindoline-1,3-dione |
| Phthalimide, N-acetyl- |
| 1H-Isoindole-1,3(2H)-dione, 2-acetyl- |
| N-Acetyl-phthalimid |
| Acetylphthalimide |
| 2-Acetyl-1H-isoindole-1,3(2H)-dione |
| MFCD00023054 |
| Phthalimide,N-acetyl |
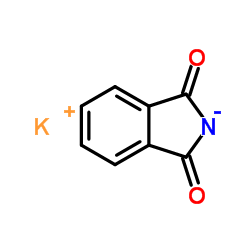 CAS#:1074-82-4
CAS#:1074-82-4 CAS#:75-36-5
CAS#:75-36-5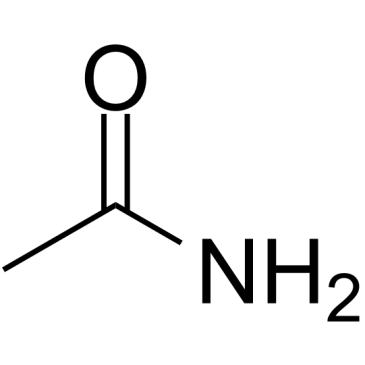 CAS#:60-35-5
CAS#:60-35-5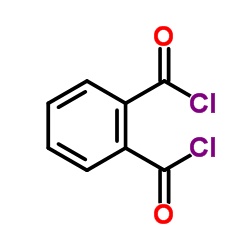 CAS#:88-95-9
CAS#:88-95-9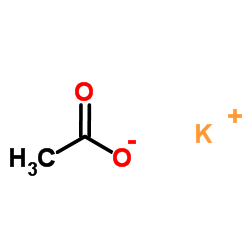 CAS#:127-08-2
CAS#:127-08-2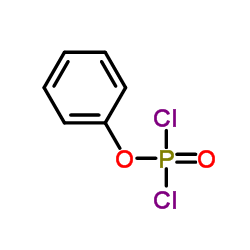 CAS#:770-12-7
CAS#:770-12-7 CAS#:80824-98-2
CAS#:80824-98-2 CAS#:64-19-7
CAS#:64-19-7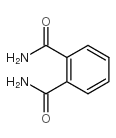 CAS#:88-96-0
CAS#:88-96-0 CAS#:934-87-2
CAS#:934-87-2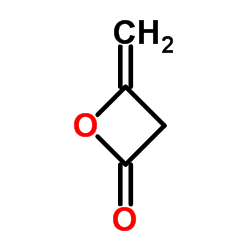 CAS#:674-82-8
CAS#:674-82-8 CAS#:122-79-2
CAS#:122-79-2 CAS#:32362-99-5
CAS#:32362-99-5 CAS#:10436-83-6
CAS#:10436-83-6 CAS#:2142-04-3
CAS#:2142-04-3 CAS#:51-66-1
CAS#:51-66-1