Sorafenib

Sorafenib structure
|
Common Name | Sorafenib | ||
|---|---|---|---|---|
| CAS Number | 284461-73-0 | Molecular Weight | 464.825 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 523.3±50.0 °C at 760 mmHg | |
| Molecular Formula | C21H16ClF3N4O3 | Melting Point | 202-204°C | |
| MSDS | N/A | Flash Point | 270.3±30.1 °C | |
Use of SorafenibSorafenib (Bay 43-9006) is a potent multikinase inhibitor with IC50s of 6 nM, 20 nM, and 22 nM for Raf-1, B-Raf, and VEGFR-3, respectively. |
| Name | sorafenib |
|---|---|
| Synonym | More Synonyms |
| Description | Sorafenib (Bay 43-9006) is a potent multikinase inhibitor with IC50s of 6 nM, 20 nM, and 22 nM for Raf-1, B-Raf, and VEGFR-3, respectively. |
|---|---|
| Related Catalog | |
| Target |
VEGFR3:20 nM (IC50) Braf:22 nM (IC50) Raf-1:6 nM (IC50) VEGFR2:90 nM (IC50) PDGFRβ:57 nM (IC50) BrafV599E:38 nM (IC50) c-Kit:68 nM (IC50) Flt3:58 nM (IC50) |
| In Vitro | Sorafenib (BAY 43-9006) also inhibits BRAFwt (IC50=22 nM), BRAFV599E (IC50=38 nM), VEGFR-2 (IC50=90 nM), VEGFR-3 (IC50=20 nM), PDGFR-β (IC50=57 nM), c-KIT (IC50=68 nM), and Flt3 (IC50=58 nM) in biochemical assays. In MDA-MB-231 breast cancer cells, Sorafenib completely blocks activation of the MAPK pathway. Cells are preincubated with Sorafenib (0.01 to 3 μM), and dose-dependent inhibition of basal MEK 1/2 and ERK 1/2 phosphorylation (IC50, 40 and 100 nM, respectively)[1]. |
| In Vivo | Sorafenib demonstrates broad oral antitumor efficacy in panel of human tumor xenograft models. Sorafenib is given orally at 7.5 to 60 mg/kg. There is no lethality and no increase in weight loss in any treated group relative to the corresponding control group. Daily oral administration of Sorafenib (30 to 60 mg/kg) produces complete tumor stasis during treatment in five of the six models[1]. The survival rate is 73.3 % in Diethyl nitrosamine (DENA) group and 83.3 % in Sorafenib group compared to 100 % in the normal control group. DENA group shows a significant increase in liver index (1.51-fold increase, p<0.05) compared to normal control group, while treatment with Sorafenib shows significant decrease (p<0.05) in liver index when compared to DENA group. The liver index in Sorafenib group significantly decreases to lower than its value in the normal control[2]. |
| Kinase Assay | To test compound inhibition against various RAF kinase isoforms, Sorafenib is added to a mixture of Raf-1 (80 ng), wt BRAF, or V599E BRAF (80 ng) with MEK-1 (1 μg) in assay buffer [20 mM Tris (pH 8.2), 100 mM NaCl, 5 mM MgCl2, and 0.15% β-mercaptoethanol] at a final concentration of 1% DMSO. The RAF kinase assay (final volume of 50 μL) is initiated by adding 25 μL of 10 μM γ-[33P]ATP (400 Ci/mol) and incubated at 32°C for 25 minutes. Phosphorylated MEK-1 is harvested by filtration onto a phosphocellulose mat, and 1% phosphoric acid is used to wash away unbound radioactivity. After drying by microwave heating, a β-plate counter is used to quantify filter-bound radioactivity[1]. |
| Cell Assay | The MDA-MB-231 human mammary adenocarcinoma cell lines are plated at 2×105 cells per well in 12-well tissue culture plates in DMEM growth media (10% heat-inactivated FCS) overnight. Cells are washed once with serum-free media and incubated in DMEM supplemented with 0.1% fatty acid-free BSA containing various concentrations of BAY 43-9006 (0.01, 0.03 , 0.1, 0.3, 1, 3 μM) in 0.1% DMSO for 120 minutes to measure changes in basal pMEK 1/2, pERK 1/2, or pPKB. Cells are washed with cold PBS (PBS containing 0.1 mM vanadate) and lysed in a 1% (v/v) Triton X-100 solution containing protease inhibitors. Lysates are clarified by centrifugation, subjected to SDS-PAGE, transferred to nitrocellulose membranes, blocked in TBS-BSA, and probed with anti-pMEK 1/2 (Ser217/Ser221; 1:1000), anti-MEK 1/2, anti-pERK 1/2 (Thr202/Tyr204; 1:1000), anti-ERK 1/2, anti-pPKB (Ser473; 1:1000), or anti-PKB primary antibodies. Blots are developed with horseradish peroxidase (HRP)-conjugated secondary antibodies and developed with Amersham ECL reagent on Amersham Hyperfilm[1]. |
| Animal Admin | Mice[1] Female NCr-nu/nu mice are used. Mice bearing 75 to 150 mg tumors are treated orally with Sorafenib (7.5 to 60 mg/kg), administered daily for 9 days. In each model, Sorafenib produces dose-dependent tumor growth inhibition with no evidence of toxicity, as measured by increased weight loss relative to control animals or drug-related lethality. In parallel to the antitumor efficacy studies, additional groups of four mice bearing 100 to 200 mg tumors are treated orally with vehicle or Sorafenib (30 to 60 mg/kg), administered daily for 5 days, which is the shortest treatment duration producing complete tumor stasis in the treated groups. Rats[2] In the study, 100- to 120-g male albino rats are utilized. After acclimatization period, rats are weighed and randomly divided into three groups: Group 1 (normal control group; n=10) is given the vehicle daily for 8 weeks. Group 2 (DENA group; n=15) receive i.p. single dose of 200 mg/kg DENA. Group 3 (Sorafenib group; n=12) is given Sorafenib orally at a dose of 10 mg/kg daily for 2 weeks, 6 weeks after DENA i.p. injection. At the end of the experiment (8 weeks), rats are weighed, anesthetized by ether, and killed, and their livers are dissected. Fresh liver is washed twice with ice-cold saline, dried on clean paper towel, and weighed. Liver index is calculated as liver weight (g)/final body weight (g)×100. The liver is divided into five portions: one portion is preserved in 10 % formalin for histopathological examination and the other portions are immediately frozen in liquid nitrogen and stored at −80°C. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 523.3±50.0 °C at 760 mmHg |
| Melting Point | 202-204°C |
| Molecular Formula | C21H16ClF3N4O3 |
| Molecular Weight | 464.825 |
| Flash Point | 270.3±30.1 °C |
| Exact Mass | 464.086304 |
| PSA | 92.35000 |
| LogP | 5.16 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.626 |
| InChIKey | MLDQJTXFUGDVEO-UHFFFAOYSA-N |
| SMILES | CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(C(F)(F)F)c3)cc2)ccn1 |
| Storage condition | -20°C Freezer |
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S26-S37/39 |
| WGK Germany | 2 |
| RTECS | HM1650000 |
| Precursor 10 | |
|---|---|
| DownStream 1 | |
| SORAFENIB TOLSYLATE |
| 2-Pyridinecarboxamide, 4-[4-[[[[4-chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]phenoxy]-N-methyl- |
| MFCD08235032 |
| Bay 43-9006 |
| Forafenib |
| 4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)phenoxy)-N-methylpicolinamide |
| Sorafenib Free Base |
| Sorafenib |
| 4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)phenoxy]-N-methylpyridine-2-carboxamide |
| 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)phenoxy]-N-methyl-2-pyridinecarboxamide |
| BAY-43-900 |
| Nexavar |
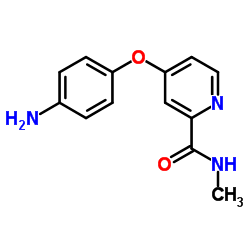 CAS#:284462-37-9
CAS#:284462-37-9 CAS#:327-78-6
CAS#:327-78-6![N-[4-chloro-3-(trifluoromethyl)phenyl]-1H-imidazole-1-carboxamide Structure](https://image.chemsrc.com/caspic/491/1187086-96-9.png) CAS#:1187086-96-9
CAS#:1187086-96-9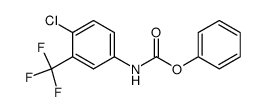 CAS#:871555-75-8
CAS#:871555-75-8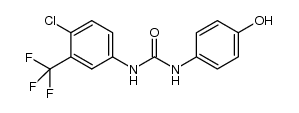 CAS#:1129683-83-5
CAS#:1129683-83-5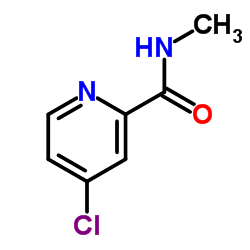 CAS#:220000-87-3
CAS#:220000-87-3![N-[4-Chloro-3-(trifluoromethyl)phenyl]urea Structure](https://image.chemsrc.com/caspic/316/343247-69-8.png) CAS#:343247-69-8
CAS#:343247-69-8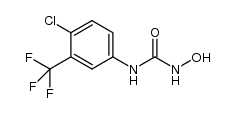 CAS#:1129683-96-0
CAS#:1129683-96-0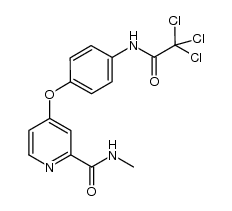 CAS#:1129683-94-8
CAS#:1129683-94-8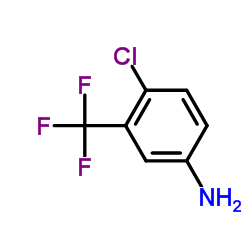 CAS#:320-51-4
CAS#:320-51-4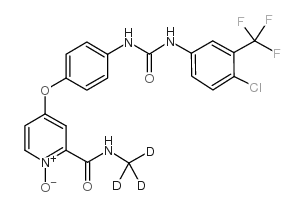 CAS#:583840-03-3
CAS#:583840-03-3
