ibuprofen sodium salt
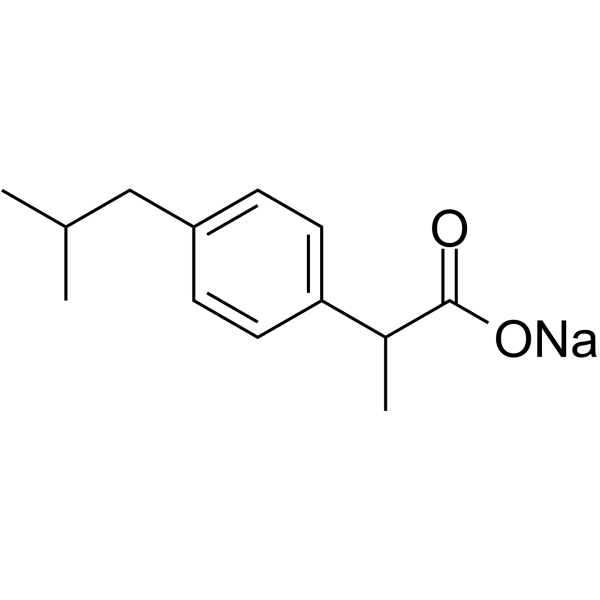
ibuprofen sodium salt structure
|
Common Name | ibuprofen sodium salt | ||
|---|---|---|---|---|
| CAS Number | 31121-93-4 | Molecular Weight | 228.26300 | |
| Density | 1.029g/cm3 | Boiling Point | 319.6ºC at 760 mmHg | |
| Molecular Formula | C13H17NaO2 | Melting Point | 75-77ºC | |
| MSDS | USA | Flash Point | 216.7ºC | |
Use of ibuprofen sodium saltIbuprofen ((±)-Ibuprofen) sodium is an orally active, selective COX-1 inhibitor with an IC50 value of 13 μM. Ibuprofen sodium inhibits cell proliferation, angiogenesis, and induces cell apoptosis. Ibuprofen sodium is a nonsteroidal anti-inflammatory agent and a nitric oxide (NO) donor. Ibuprofen sodium can be used in the research of pain, swelling, inflammation, infection, immunology, cancers[1][2][5][8]. |
| Name | sodium,2-[4-(2-methylpropyl)phenyl]propanoate |
|---|---|
| Synonym | More Synonyms |
| Description | Ibuprofen ((±)-Ibuprofen) sodium is an orally active, selective COX-1 inhibitor with an IC50 value of 13 μM. Ibuprofen sodium inhibits cell proliferation, angiogenesis, and induces cell apoptosis. Ibuprofen sodium is a nonsteroidal anti-inflammatory agent and a nitric oxide (NO) donor. Ibuprofen sodium can be used in the research of pain, swelling, inflammation, infection, immunology, cancers[1][2][5][8]. |
|---|---|
| Related Catalog | |
| Target |
COX-1:13 μM (IC50) COX-2:370 μM (IC50) |
| In Vitro | Ibuprofen sodium (24 h) inhibits COX-1 and COX-2 activity with IC50 values of 13 μM and 370 μM[1]. Ibuprofen sodium (500 μM, 48 h) inhibits cell proliferation and angiogenesis, and induces apoptosis in AGS cells (Adenocarcinoma gastric cell line)[2]. Ibuprofen sodium (500 μM, 48 h) downregulates transcription of Akt, VEGF-A, PCNA, Bcl2, OCT3/4 and CD44 genes, but upregulates RNA levels of wild type P53 and Bax genes in AGS cell[2]. Ibuprofen sodium (500 μM, 24 h) restores microtubule reformation, microtubule-dependent intracellular cholesterol transport, and induces extension of microtubules to the cell periphery in both cystic fibrosis (CF) cell models and primary CF nasal epithelial cells[3]. Ibuprofen sodium (500 μM, 24 h) enhances UV-induced cell death in MCF-7 cells and MDA-MB-231 cells by a photosensitization process[4]. Cell Viability Assay[2] Cell Line: AGS cells Concentration: 100-1000 μM Incubation Time: 24 h, 48 h Result: Inhibited AGS cell viability with IC50 values of 630 μM (trypan blue staining, 24 h), 456 μM (neutral red assay, 24 h), 549 μM (trypan blue staining , 48 h) and 408 μM (neutral red assay, 48 h). |
| In Vivo | Ibuprofen sodium (fed in animal feedings, 300 mg/kg, 14 days) reduces overall tumor growth and enhances anti-tumor immune characteristics without adverse autoimmune reactions in a model of postpartum breast cancer[5]. Ibuprofen sodium (subcutaneous injection, 60 mg/kg, every second day for 15 days) reduces the risk of neuropathy in a rat model of chronic Oxaliplatin‑induced peripheral neuropathy[6]. Ibuprofen sodium (oral administration, 20 mg/kg, every 12 hours, 5 doses total) decreases muscle growth (average muscle fiber cross-sectional area) without affecting regulation of supraspinatus tendon adaptions to exercise[7]. Ibuprofen sodium (oral administration, 35 mg/kg, twice daily) attenuates the Inflammatory response to pseudomonas aeruginosa in a rat model of chronic pulmonary infection[8]. Animal Model: Syngeneic (D2A1) orthotopic Balb/c mouse model of PPBC (postpartum)[5] Dosage: 300 mg/kg, daily for 14 days Administration: Fed in animal feedings (added to pulverized standard chow and mixed dry, then mixed with water, made into chow pellets and dried thoroughly) Result: Suppresed tumor growth, reduced presence of immature monocytes and increased numbers of T cells. Enhanced Th1 associated cytokines as well as promoted tumor border accumulation of T cells. Animal Model: Oxaliplatin‑induced peripheral neuropathy[6] Dosage: 60 mg/kg, every second day for 15 days Administration: Subcutaneous injection Result: Lowered sensory nerve conduction velocity (SNCV). |
| References |
| Density | 1.029g/cm3 |
|---|---|
| Boiling Point | 319.6ºC at 760 mmHg |
| Melting Point | 75-77ºC |
| Molecular Formula | C13H17NaO2 |
| Molecular Weight | 228.26300 |
| Flash Point | 216.7ºC |
| Exact Mass | 228.11300 |
| PSA | 40.13000 |
| LogP | 1.73850 |
| InChIKey | PTTPUWGBPLLBKW-UHFFFAOYSA-M |
| SMILES | CC(C)Cc1ccc(C(C)C(=O)[O-])cc1.[Na+] |
| Storage condition | 2-8°C |
| Stability | Stable. Incompatible with strong oxidizing agents |
| Water Solubility | H2O: 100 mg/mL, may be clear to slightly hazy |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | 22-24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | MU6641000 |
| HS Code | 2916399090 |
|
~94% 
ibuprofen sodiu... CAS#:31121-93-4 |
| Literature: WO2011/1228 A1, ; Page/Page column 13 ; |
|
~82% 
ibuprofen sodiu... CAS#:31121-93-4 |
| Literature: US5696165 A1, ; |
| HS Code | 2916399090 |
|---|---|
| Summary | 2916399090 other aromatic monocarboxylic acids, their anhydrides, halides, peroxides, peroxyacids and their derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
The advantages and limitations of the analgesics available for control of postoperative pain after a dental procedure.
SAAD Dig. 29 , 70-81, (2013)
|
|
|
Enhanced longevity by ibuprofen, conserved in multiple species, occurs in yeast through inhibition of tryptophan import.
PLoS Genet. 10(12) , e1004860, (2014) The common non-steroidal anti-inflammatory drug ibuprofen has been associated with a reduced risk of some age-related pathologies. However, a general pro-longevity role for ibuprofen and its mechanist... |
|
|
Pegylation improves the pharmacokinetics and bioavailability of small-molecule drugs hydrolyzable by esterases: a study of phospho-Ibuprofen.
J. Pharmacol. Exp. Ther. 351(1) , 61-6, (2014) Esterase hydrolysis of drugs can accelerate their elimination, thereby limiting their efficacy. Polyethylene glycol (PEG) covalently attached to drugs (pegylation) is known to improve the efficiency o... |
| EINECS 250-477-6 |
| Sodium ibuprofen |
| sodium 2-[4-(2-methylpropyl)phenyl]propanoate |
| p-Isobutylhydratropic acid sodium salt |
| 2-(4'-isobutylphenyl)propionic acid,sodium salt |
| Sodium 2-(4-isobutylphenyl)propionate |
| Hydratropic acid,p-isobutyl-,sodium salt |
| Ibuprofen sodium salt |
| Ibuprofen sodium |
| (RS)-ibuprofen sodium |
| 2-(p-isobutylphenyl)propionic acid sodium salt |

 CAS#:36039-36-8
CAS#:36039-36-8