H-Glu(OtBu)-OtBu.HCl
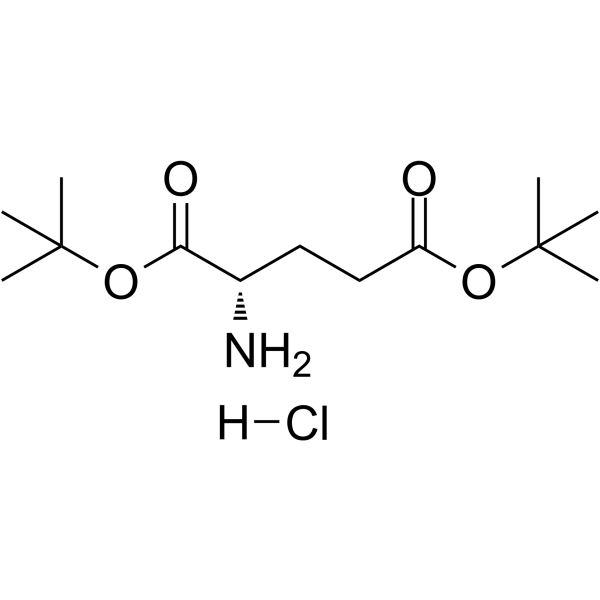
H-Glu(OtBu)-OtBu.HCl structure
|
Common Name | H-Glu(OtBu)-OtBu.HCl | ||
|---|---|---|---|---|
| CAS Number | 32677-01-3 | Molecular Weight | 295.803 | |
| Density | 1.02g/cm3 | Boiling Point | 311.1ºC at 760 mmHg | |
| Molecular Formula | C13H26ClNO4 | Melting Point | 60 to 75ºC | |
| MSDS | USA | Flash Point | 78.1ºC | |
Use of H-Glu(OtBu)-OtBu.HClH-Glu(OtBu)-OtBu hydrochloride is a glutamate derivative that can be used for substance P antagonist synthesis[1]. |
| Name | ditert-butyl (2S)-2-aminopentanedioate,hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | H-Glu(OtBu)-OtBu hydrochloride is a glutamate derivative that can be used for substance P antagonist synthesis[1]. |
|---|---|
| Related Catalog | |
| Target |
Substance P[1] |
| References |
| Density | 1.02g/cm3 |
|---|---|
| Boiling Point | 311.1ºC at 760 mmHg |
| Melting Point | 60 to 75ºC |
| Molecular Formula | C13H26ClNO4 |
| Molecular Weight | 295.803 |
| Flash Point | 78.1ºC |
| Exact Mass | 295.155029 |
| PSA | 78.62000 |
| LogP | 3.27960 |
| InChIKey | LFEYMWCCUAOUKZ-FVGYRXGTSA-N |
| SMILES | CC(C)(C)OC(=O)CCC(N)C(=O)OC(C)(C)C.Cl |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | MA0880000 |
|
~84% 
H-Glu(OtBu)-OtBu.HCl CAS#:32677-01-3 |
| Literature: US2004/87813 A1, ; |
|
~% 
H-Glu(OtBu)-OtBu.HCl CAS#:32677-01-3 |
| Literature: Recueil des Travaux Chimiques des Pays Bas, , vol. 102, # 11 p. 469 - 474 |
|
~40% 
H-Glu(OtBu)-OtBu.HCl CAS#:32677-01-3 |
| Literature: Journal of Medicinal Chemistry, , vol. 37, # 20 p. 3294 - 3302 |
| Precursor 5 | |
|---|---|
| DownStream 8 | |
|
Synthesis and biological activity of analogues of the C-terminal hexapeptide of substance P with modifications at glutaminyl and methioninyl residues. Structure-activity studies.
Int. J. Pept. Protein Res. 40 , 395-400, (1992) Analogues of [Orn6]-SP6-11 have been synthesized in which the methionyl residue is replaced by glutamine gamma-carboxamide substituted derivatives. These analogues where tested in three in vitro prepa... |
|
|
Synthesis of potent antagonists of substance P by modifying the methionyl and glutaminyl residues of its C-terminal hexapeptide and without using D-amino acids.
Int. J. Pept. Protein Res. 41 , 411-414, (1993) Analogues of [Orn6]-SP6-11 have been synthesized in which the Met11-NH2 residue is replaced by the alpha, gamma-dimethyl, alpha, gamma-dibenzyl and alpha, gamma-di-tert-butyl esters of glutamic acid. ... |
|
|
Convulsant properties of L-glutamic acid di-tert butyl ester.
Neurobehav. Toxicol. Teratol. 7 , 275-278, (1985) Glutamic acid di-tert butyl ester (GTBE) was found to have a pronounced convulsant effect in mice and rats, producing recurrent clonic convulsions combined with postural and respiratory disturbances i... |
| H-Glu(OtBu)-OtBu·HCl |
| H-Glu(OtBu)-OtBu��HCl |
| L-glutamic acid di-t-butyl ester hydrochloride |
| MFCD00058003 |
| Di-tert-butyl L-glutamate hydrochloride (1:1) |
| L-Glutamic acid, bis(1,1-dimethylethyl) ester, hydrochloride (1:1) |
| H-Glu(OtBu)-OtBu,HCl |
| H-L-Glu(OtBu)-OtBu*HCl |
| L-Glutamic acid di-tert |
| L-Glutamic Acid di-Tert-Butyl Ester Hydrochloride |
| Bis(2-methyl-2-propanyl) L-glutamate hydrochloride (1:1) |
| (S)-Di-tert-butyl 2-aminopentanedioate hydrochloride |
| H-Glu(OtBu)-OtBu.HCl |
| L-Glutamic acid di-tert-butylester hydrochloride |
![Bis(2-methyl-2-propanyl) N-[(benzyloxy)carbonyl]-L-glutamate structure](https://image.chemsrc.com/caspic/204/16881-41-7.png)

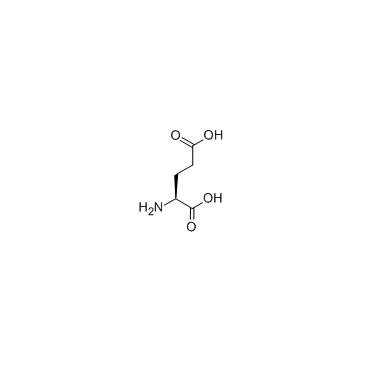 CAS#:56-86-0
CAS#:56-86-0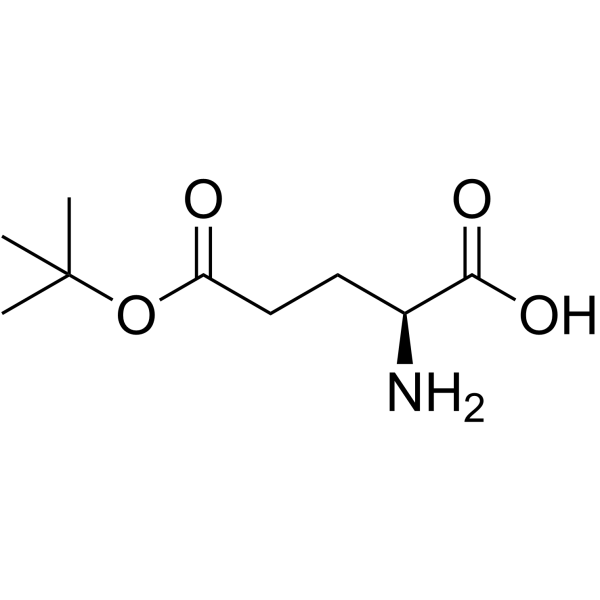 CAS#:2419-56-9
CAS#:2419-56-9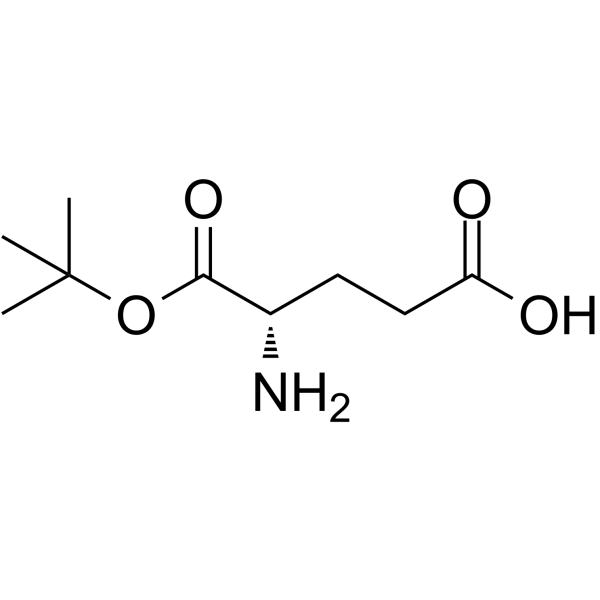 CAS#:45120-30-7
CAS#:45120-30-7![N-[(4-Methylphenyl)sulfonyl]-L-glutamic acid structure](https://image.chemsrc.com/caspic/265/4816-80-2.png) CAS#:4816-80-2
CAS#:4816-80-2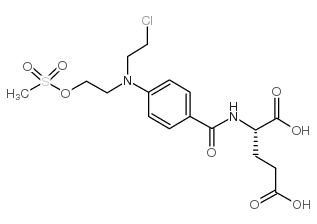 CAS#:122665-73-0
CAS#:122665-73-0 CAS#:3086-06-4
CAS#:3086-06-4![ditert-butyl 2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methyl-amino]benzoyl]amino]pentanedioate structure](https://image.chemsrc.com/caspic/063/86669-33-2.png) CAS#:86669-33-2
CAS#:86669-33-2
