5-Methoxyindole-3-acetic acid
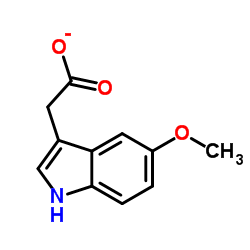
5-Methoxyindole-3-acetic acid structure
|
Common Name | 5-Methoxyindole-3-acetic acid | ||
|---|---|---|---|---|
| CAS Number | 3471-31-6 | Molecular Weight | 205.21000 | |
| Density | N/A | Boiling Point | 445.9ºC at 760 mmHg | |
| Molecular Formula | C11H11NO3 | Melting Point | 145-148 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 223.5ºC | |
Use of 5-Methoxyindole-3-acetic acid5-Methoxyindole-3-acetic acid, isolated from pineal tissue, is a metabolite of Melatonin[1]. |
| Name | 2-(5-methoxy-1H-indol-3-yl)acetic acid |
|---|---|
| Synonym | More Synonyms |
| Description | 5-Methoxyindole-3-acetic acid, isolated from pineal tissue, is a metabolite of Melatonin[1]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Boiling Point | 445.9ºC at 760 mmHg |
|---|---|
| Melting Point | 145-148 °C (dec.)(lit.) |
| Molecular Formula | C11H11NO3 |
| Molecular Weight | 205.21000 |
| Flash Point | 223.5ºC |
| Exact Mass | 205.07400 |
| PSA | 62.32000 |
| LogP | 1.80360 |
| InChIKey | COCNDHOPIHDTHK-UHFFFAOYSA-N |
| SMILES | COc1ccc2[nH]cc(CC(=O)O)c2c1 |
| Storage condition | 2-8°C |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 10 | |
|---|---|
| DownStream 8 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Indoleamines and 5-methoxyindoles in trout pineal organ in vivo: daily changes and influence of photoperiod.
Gen. Comp. Endocrinol. 144(1) , 67-77, (2005) This study describes the diel rhythms in several indoleamines, melatonin, and related 5-methoxyindoles in the pineal organ of rainbow trout in vivo. In addition, the effect of different photoperiod co... |
|
|
Total pineal endocrine substitution therapy (TPEST) as a new neuroendocrine palliative treatment of untreatable metastatic solid tumor patients: a phase II study.
Neuro Endocrinol. Lett. 24(3-4) , 259-62, (2003) It is known since many years that the pineal gland plays an anticancer role, and melatonin (MLT), the most investigated pineal hormone, has been proven to exert antitumor activity. However, MLT would ... |
|
|
Secretion of the methoxyindoles melatonin, 5-methoxytryptophol, 5-methoxyindoleacetic acid, and 5-methoxytryptamine from trout pineal organs in superfusion culture: effects of light intensity.
Gen. Comp. Endocrinol. 101(2) , 165-72, (1996) The teleost pineal organ is a photoreceptive endocrine organ that synthesizes the hormone melatonin through specialized intrapineal photoreceptor cells in a light-dependent manner. The present study i... |
| 5-Methoxy-3-indoleacetic acid |
| 5-methoxy-1H-indole-3-acetic acid |
| 5-Methoxyindole-3-aceticacid |
| 2-(5-Methoxy-3-indolyl)acetic acid |
| 5-Methoxyindoleacetate |
| 5-Methoxyindoleacetic acid |
| MFCD00005638 |
| EINECS 222-438-3 |
| 1H-Indole-3-acetic acid, 5-methoxy-, ion(1-) |
| 5-Methoxyindol-3-ylacetate |
| INDOLE-3-ACETIC ACID,5-METHOXY |
| (5-Methoxy-1H-indol-3-yl)acetate |
| 5-Methoxyindol-3-ylacetic acid |
| 5-Methoxyindole-3-acetate |
| 5-Methoxyindole-3-acetic acid |
| 2-(5-Methoxy-1H-indol-3-yl)acetic acid |
| Methoxyindoleacetic acid |
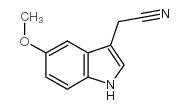 CAS#:2436-17-1
CAS#:2436-17-1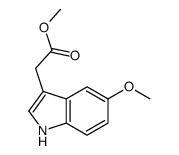 CAS#:23304-48-5
CAS#:23304-48-5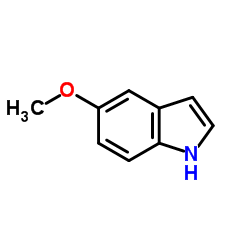 CAS#:1006-94-6
CAS#:1006-94-6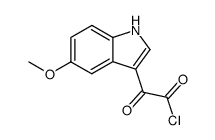 CAS#:2426-19-9
CAS#:2426-19-9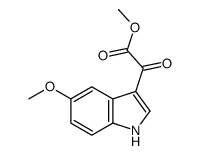 CAS#:99988-56-4
CAS#:99988-56-4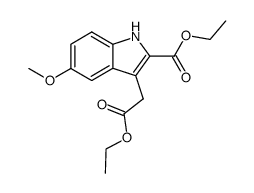 CAS#:72963-98-5
CAS#:72963-98-5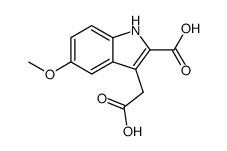 CAS#:858232-58-3
CAS#:858232-58-3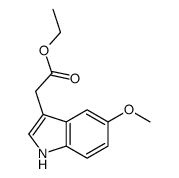 CAS#:57000-49-4
CAS#:57000-49-4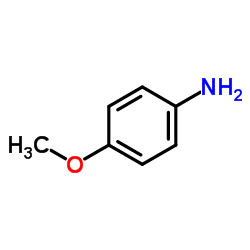 CAS#:104-94-9
CAS#:104-94-9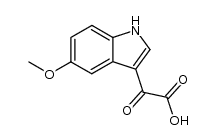 CAS#:14827-68-0
CAS#:14827-68-0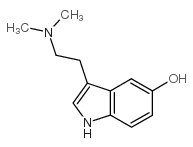 CAS#:487-93-4
CAS#:487-93-4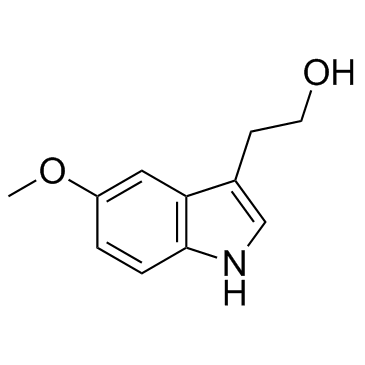 CAS#:712-09-4
CAS#:712-09-4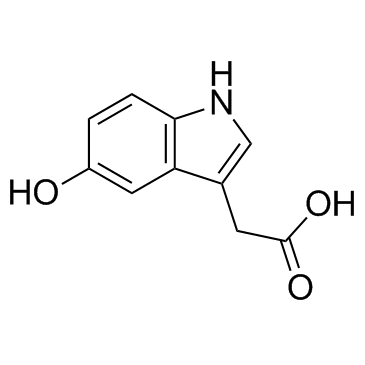 CAS#:54-16-0
CAS#:54-16-0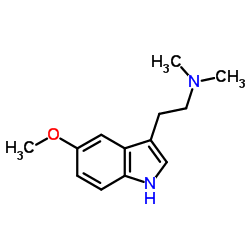 CAS#:1019-45-0
CAS#:1019-45-0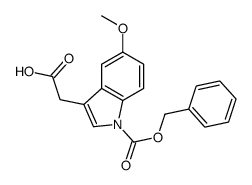 CAS#:924635-04-1
CAS#:924635-04-1 CAS#:55887-55-3
CAS#:55887-55-3
