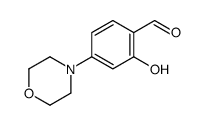
1-(2-hydroxy-4-morpholin-4-ylphenyl)ethanone
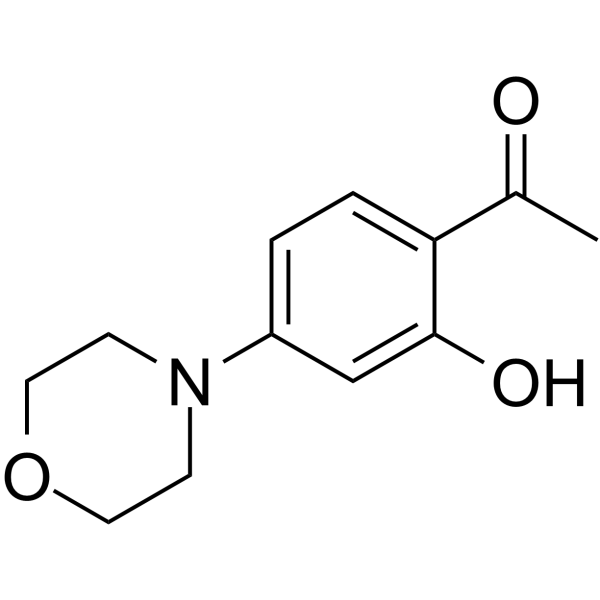
1-(2-hydroxy-4-morpholin-4-ylphenyl)ethanone structure
|
Common Name | 1-(2-hydroxy-4-morpholin-4-ylphenyl)ethanone | ||
|---|---|---|---|---|
| CAS Number | 404009-40-1 | Molecular Weight | 221.252 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 425.5±45.0 °C at 760 mmHg | |
| Molecular Formula | C12H15NO3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 211.1±28.7 °C | |
Use of 1-(2-hydroxy-4-morpholin-4-ylphenyl)ethanoneIC 86621 is a potent DNA-dependent protein kinase (DNA-PK) inhibitor, with an IC50 of 120 nM. IC 86621 also acts as a selective and reversible ATP-competitive inhibitor.IC 86621 inhibits DNA-PK mediated cellular DNA double-strand break (DSB) repair (EC50=68 µM). IC 86621 increases DSB-induced antitumor activity without cytotoxic effects. IC 86621 can protects rheumatoid arthritis (RA) T cells from apoptosis[1][2]. |
| Name | 1-(2-hydroxy-4-morpholin-4-ylphenyl)ethanone |
|---|---|
| Synonym | More Synonyms |
| Description | IC 86621 is a potent DNA-dependent protein kinase (DNA-PK) inhibitor, with an IC50 of 120 nM. IC 86621 also acts as a selective and reversible ATP-competitive inhibitor.IC 86621 inhibits DNA-PK mediated cellular DNA double-strand break (DSB) repair (EC50=68 µM). IC 86621 increases DSB-induced antitumor activity without cytotoxic effects. IC 86621 can protects rheumatoid arthritis (RA) T cells from apoptosis[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 120 nM (DNA-PK)[1] |
| In Vitro | IC 86621 exhibits high selectivity against other kinases such as PI3K, Cdk2, Src, PKA, PKC, Chk1, CK1, ATM, and FKBP12[1]. IC 86621 (0-100 nM, 24 h) protects RA (rheumatoid arthritis) T cells from apoptosis[2]. Apoptosis Analysis[2] Cell Line: CD4+CD45RO- T cells (from six control donors and seven RA patients) Concentration: 0 nM, 50 nM, 100 nM Incubation Time: 24 h Result: Protected RA (rheumatoid arthritis) T cells from apoptosis. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 425.5±45.0 °C at 760 mmHg |
| Molecular Formula | C12H15NO3 |
| Molecular Weight | 221.252 |
| Flash Point | 211.1±28.7 °C |
| Exact Mass | 221.105194 |
| PSA | 49.77000 |
| LogP | 1.40 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.570 |
| InChIKey | YHKSBKQXCWHTQL-UHFFFAOYSA-N |
| SMILES | CC(=O)c1ccc(N2CCOCC2)cc1O |
| Storage condition | -20℃ |
| HS Code | 2934999090 |
|---|
|
~25% 
1-(2-hydroxy-4-... CAS#:404009-40-1 |
| Literature: Halbrook, James; Kesicki, Edward A.; Burgess, Laurence E.; Schlachter, Stephen T.; Eary, Charles T.; Schiro, Justin G; Huang, Hongmei; Evans, Michael; Han, Yongxin Patent: US2002/165218 A1, 2002 ; US 20020165218 A1 |
|
~17% 
1-(2-hydroxy-4-... CAS#:404009-40-1 |
| Literature: Knight, Zachary A.; Chiang, Gary G.; Alaimo, Peter J.; Kenski, Denise M.; Ho, Caroline B.; Coan, Kristin; Abraham, Robert T.; Shokat, Kevan M. Bioorganic and Medicinal Chemistry, 2004 , vol. 12, # 17 p. 4749 - 4759 |
|
~% 
1-(2-hydroxy-4-... CAS#:404009-40-1 |
| Literature: Knight, Zachary A.; Chiang, Gary G.; Alaimo, Peter J.; Kenski, Denise M.; Ho, Caroline B.; Coan, Kristin; Abraham, Robert T.; Shokat, Kevan M. Bioorganic and Medicinal Chemistry, 2004 , vol. 12, # 17 p. 4749 - 4759 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| 1-[2-Hydroxy-4-(4-morpholinyl)phenyl]ethanone |
| AR2783 |
| 1-(2-Hydroxy-4-morpholin-4-yl-phenyl)ethanone |
| DNA-PK Inhibitor III |
| HMS3229C13 |
| 1-(2-hydroxy-4-morpholin-4-ylphenyl)ethanone |
| Ethanone, 1-[2-hydroxy-4-(4-morpholinyl)phenyl]- |