Hecogenin
Modify Date: 2024-01-02 10:20:04
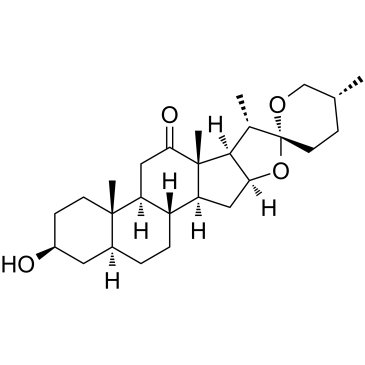
Hecogenin structure
|
Common Name | Hecogenin | ||
|---|---|---|---|---|
| CAS Number | 467-55-0 | Molecular Weight | 430.620 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 548.9±50.0 °C at 760 mmHg | |
| Molecular Formula | C27H42O4 | Melting Point | 268°C | |
| MSDS | N/A | Flash Point | 177.8±23.6 °C | |
Use of HecogeninHecogenin is a steroid saponin isolated from Agave sisalana and is a selective inhibitor of human UDP-glucuronosyltransferases. Hecogenin has a wide spectrum of pharmacological activities, including anti-inflammatory, antifungal and gastroprotective effects[1]. |
| Name | Hecogenin |
|---|---|
| Synonym | More Synonyms |
| Description | Hecogenin is a steroid saponin isolated from Agave sisalana and is a selective inhibitor of human UDP-glucuronosyltransferases. Hecogenin has a wide spectrum of pharmacological activities, including anti-inflammatory, antifungal and gastroprotective effects[1]. |
|---|---|
| Related Catalog | |
| Target |
Human UDP-glucuronosyltransferases[1] |
| In Vivo | Hecogenin (3.1-90 mg/kg; oral administration; for 15 hours; male Swiss mice) acutely administered, before ethanol, exhibits a potent gastroprotective effect. The Hecogenin pretreatment normalizes GSH levels and significantly reduces lipid peroxidation and nitrite levels in the stomach, as evaluated by the ethanol-induced gastric lesion model. Hecogenin also decreases MPO release and significantly protects the gastric mucosa[1]. Animal Model: Male Swiss mice (20-30 g) with ethanol[1] Dosage: 3.1 mg/kg, 7.5 mg/kg, 15 mg/kg, 30 mg/kg, 60 mg/kg and 90 mg/kg Administration: Oral administration; for 15 hours Result: Normalized GSH levels and significantly reduced lipid peroxidation and nitrite levels in the stomach, as evaluated by the ethanol-induced gastric lesion model. Decreased MPO release and significantly protected the gastric mucosa. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 548.9±50.0 °C at 760 mmHg |
| Melting Point | 268°C |
| Molecular Formula | C27H42O4 |
| Molecular Weight | 430.620 |
| Flash Point | 177.8±23.6 °C |
| Exact Mass | 430.308319 |
| PSA | 55.76000 |
| LogP | 4.22 |
| Vapour Pressure | 0.0±3.3 mmHg at 25°C |
| Index of Refraction | 1.559 |
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S26-S36 |
| WGK Germany | 3 |
| Precursor 7 | |
|---|---|
| DownStream 6 | |
| 5ALPHA-SPIROSTAN-3BETA-OL-12-ONE |
| 5α-Spirostan-12-one, 3β-hydroxy-, (25R)- |
| (25R)-5-Spirostan-3-ol-12-one |
| MFCD00067285 |
| (22R,25R)-3β-Hydroxy-5α-spirostan-12-one |
| Spirostan-12-one, 3-hydroxy-, (3β,5α,25R)- |
| 12-Oxotigogenin |
| (3β,5α,25R)-3-Hydroxyspirostan-12-one |
| Hocogenin |
| Hecoginin |
| (25R)-3b-Hydroxy-5a-spirostan-12-one |
| Hecogenin |
| EINECS 207-392-4 |
| 3-b-Hydroxy-5-a-spirostan-12-one |
| (3,5,25R)-3-Hydroxysiprostan-1 |
| (3,5,25R)-3-Hydroxysiprostan-12-one |
| (3b,5a,25R)-3-Hydroxyspirostan-12-one |
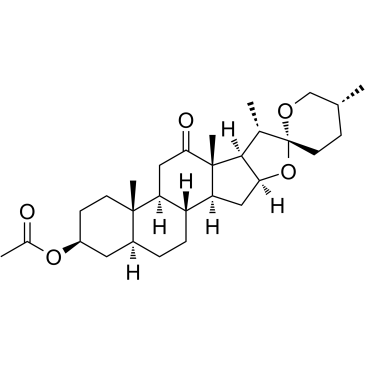 CAS#:915-35-5
CAS#:915-35-5 CAS#:2137-20-4
CAS#:2137-20-4 CAS#:6875-60-1
CAS#:6875-60-1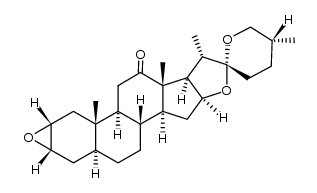 CAS#:28180-63-4
CAS#:28180-63-4 CAS#:108747-55-3
CAS#:108747-55-3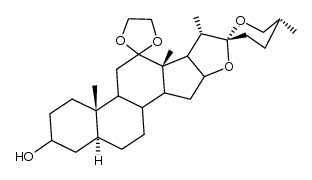 CAS#:38673-31-3
CAS#:38673-31-3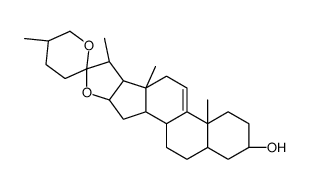 CAS#:1106-20-3
CAS#:1106-20-3 CAS#:378-44-9
CAS#:378-44-9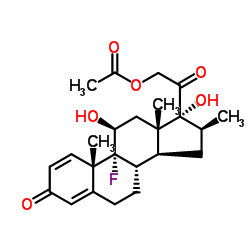 CAS#:987-24-6
CAS#:987-24-6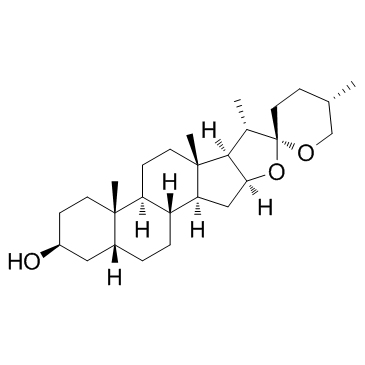 CAS#:126-19-2
CAS#:126-19-2
