Cortisone acetate
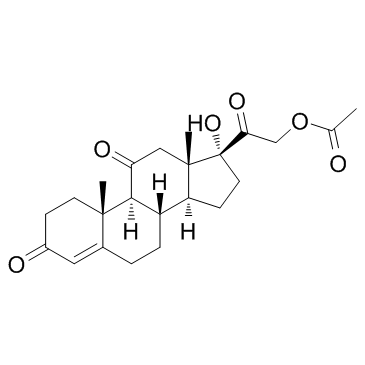
Cortisone acetate structure
|
Common Name | Cortisone acetate | ||
|---|---|---|---|---|
| CAS Number | 50-04-4 | Molecular Weight | 402.481 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 577.3±50.0 °C at 760 mmHg | |
| Molecular Formula | C23H30O6 | Melting Point | 237-240 °C(lit.) | |
| MSDS | USA | Flash Point | 197.3±23.6 °C | |
Use of Cortisone acetateCortisone acetate (17-hydroxy-11-dehydrocorticosterone), a 21-carbon steroid hormone, is one of the main hormones released by the adrenal gland in response to stress.IC50 Value: Target: Glucocorticoid Receptorin vitro: Cortisone suppressed this apoptosis at a concentration range of 1-10,000 ng/ml (2.8-28,000 nM) dose-dependently. Suppression of cortisol-induced apoptosis by cortisonewas consistently observed in PBMCs derived from 16 healthy subjects. Examination for inhibitory activities of the steroids against [3H]dexamethasone binding to PBMCs suggested that cortisone can bind cellular GC-receptors (GC-Rs), but the affinity of cortisone to GCRs is 1/30 or less than that of cortisol [1]. Apoptosis was also readily induced in primary cultures of third trimester decidual cells when treated with cortisol, cortisone, or dexamethasone (all 100 nM for 24 h) [2]. in vivo: The effects of a single dose of cortisone acetate (5 or 10 mg/100 g body weight) on B cells were examined in young chickens. A dose-dependent increase in numbers of circulating B lymphocytes and a change in their Ig-class distribution were followed by parallel increase in splenic plasma cells and serum immunoglobulins. The higher dose of cortisone produced changes in Bmu and Bgamma cells, whereas the lower dose primarily affected Bmu cells [3]. Adult female CD-1 mice received daily injections of cortisone acetate (0--50 mg/kg subcutaneously) and/or amphotericin B (0--12.5 mg/kg intraperitoneally) in a checkerboard combination dosage pattern for 30 days. Dosages of amphotericin B and cortisone acetate that produced little or no mortality individually produced significant (P less than 0.005) mortality in combination [4].Toxicity: Oral use of cortisone has a number of potential systemic side-effects: hyperglycemia, insulin resistance, diabetes mellitus, osteoporosis, anxiety, depression, amenorrhoea, cataracts and glaucoma, among other problems. |
| Name | Cortisone acetate |
|---|---|
| Synonym | More Synonyms |
| Description | Cortisone acetate (17-hydroxy-11-dehydrocorticosterone), a 21-carbon steroid hormone, is one of the main hormones released by the adrenal gland in response to stress.IC50 Value: Target: Glucocorticoid Receptorin vitro: Cortisone suppressed this apoptosis at a concentration range of 1-10,000 ng/ml (2.8-28,000 nM) dose-dependently. Suppression of cortisol-induced apoptosis by cortisonewas consistently observed in PBMCs derived from 16 healthy subjects. Examination for inhibitory activities of the steroids against [3H]dexamethasone binding to PBMCs suggested that cortisone can bind cellular GC-receptors (GC-Rs), but the affinity of cortisone to GCRs is 1/30 or less than that of cortisol [1]. Apoptosis was also readily induced in primary cultures of third trimester decidual cells when treated with cortisol, cortisone, or dexamethasone (all 100 nM for 24 h) [2]. in vivo: The effects of a single dose of cortisone acetate (5 or 10 mg/100 g body weight) on B cells were examined in young chickens. A dose-dependent increase in numbers of circulating B lymphocytes and a change in their Ig-class distribution were followed by parallel increase in splenic plasma cells and serum immunoglobulins. The higher dose of cortisone produced changes in Bmu and Bgamma cells, whereas the lower dose primarily affected Bmu cells [3]. Adult female CD-1 mice received daily injections of cortisone acetate (0--50 mg/kg subcutaneously) and/or amphotericin B (0--12.5 mg/kg intraperitoneally) in a checkerboard combination dosage pattern for 30 days. Dosages of amphotericin B and cortisone acetate that produced little or no mortality individually produced significant (P less than 0.005) mortality in combination [4].Toxicity: Oral use of cortisone has a number of potential systemic side-effects: hyperglycemia, insulin resistance, diabetes mellitus, osteoporosis, anxiety, depression, amenorrhoea, cataracts and glaucoma, among other problems. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 577.3±50.0 °C at 760 mmHg |
| Melting Point | 237-240 °C(lit.) |
| Molecular Formula | C23H30O6 |
| Molecular Weight | 402.481 |
| Flash Point | 197.3±23.6 °C |
| Exact Mass | 402.204254 |
| PSA | 97.74000 |
| LogP | 2.53 |
| Vapour Pressure | 0.0±3.6 mmHg at 25°C |
| Index of Refraction | 1.566 |
| InChIKey | ITRJWOMZKQRYTA-RFZYENFJSA-N |
| SMILES | CC(=O)OCC(=O)C1(O)CCC2C3CCC4=CC(=O)CCC4(C)C3C(=O)CC21C |
| Stability | Stable. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Risk Phrases | R22;R36/37 |
| Safety Phrases | S22-S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | GM9140000 |
|
~% 
Cortisone acetate CAS#:50-04-4 |
| Literature: Kritchevsky et al. Journal of the American Chemical Society, 1952 , vol. 74, p. 483,485 |
|
~% 
Cortisone acetate CAS#:50-04-4 |
| Literature: Poos et al. Journal of the American Chemical Society, 1954 , vol. 76, p. 5031,5033 |
|
~% 
Cortisone acetate CAS#:50-04-4 |
| Literature: Mason; Sprague Journal of Biological Chemistry, 1948 , vol. 175, p. 451,455 Full Text Show Details Reichstein Helvetica Chimica Acta, 1937 , vol. 20, p. 978,987 |
|
~% 
Cortisone acetate CAS#:50-04-4 |
| Literature: Mason; Sprague Journal of Biological Chemistry, 1948 , vol. 175, p. 451,455 Full Text Show Details Reichstein Helvetica Chimica Acta, 1937 , vol. 20, p. 978,987 |
|
~% 
Cortisone acetate CAS#:50-04-4 |
| Literature: Mason; Sprague Journal of Biological Chemistry, 1948 , vol. 175, p. 451,455 Full Text Show Details Reichstein Helvetica Chimica Acta, 1937 , vol. 20, p. 978,987 |
|
~% 
Cortisone acetate CAS#:50-04-4 |
| Literature: Kane; Tsuji Journal of Pharmaceutical Sciences, 1983 , vol. 72, # 1 p. 30 - 35 |
|
~% 
Cortisone acetate CAS#:50-04-4 |
| Literature: Sarett Journal of Biological Chemistry, 1946 , vol. 162, p. 601,6307 |
| Precursor 5 | |
|---|---|
| DownStream 8 | |
|
Anti-angiogenic effect of Nelumbo nucifera leaf extracts in human umbilical vein endothelial cells with antioxidant potential.
PLoS ONE 10(2) , e0118552, (2015) Nelumbo nucifera Gaertn (Nymphaeaceae) has long been used as a traditional herb in Chinese, Japanese, Indian, and Korean medicinal practices since prehistoric times and flourishes today as the primary... |
|
|
Novel Aggregation Properties of Candida albicans Secreted Aspartyl Proteinase Sap6 Mediate Virulence in Oral Candidiasis.
Infect. Immun. 83 , 2614-26, (2015) Candida albicans, a commensal fungus of the oral microbiome, causes oral candidiasis in humans with localized or systemic immune deficiencies. Secreted aspartic proteinases (Saps) are a family of 10 r... |
|
|
The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps.
PLoS Pathog. 11 , e1005187, (2015) Of the over 250 Aspergillus species, Aspergillus fumigatus accounts for up to 80% of invasive human infections. A. fumigatus produces galactosaminogalactan (GAG), an exopolysaccharide composed of gala... |
| MFCD00003609 |
| 17α,21-Dihydroxy-4-pregnene-3,11,20-trione 21-acetate |
| Pregn-4-ene-3,11,20-trione, 21- (acetyloxy)-17-hydroxy- |
| 2-[(8S,9S,10R,13S,14S,17R)-17-hydroxy-10,13-dimethyl-3,11-dioxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl acetate |
| 21-Acetoxy-4-pregnen-17α-ol-3,11,20-trione |
| 4-Pregnene-17α,21-diol-3,11,20-trione 21-acetate |
| Cortisone, 21-acetate |
| [2-[(8S,9S,10R,13S,14S,17R)-17-hydroxy-10,13-dimethyl-3,11-dioxo-1,2,6,7,8,9,12,14,15,16-decahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] acetate |
| acétate de 2-[(8S,9S,10R,13S,14S,17R)-17-hydroxy-10,13-diméthyl-3,11-dioxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tétradécahydro-1H-cyclopenta[a]phénanthrén-17-yl]-2-oxoéthyle |
| EINECS 200-006-5 |
| 17-Hydroxy-3,11,20-trioxopregn-4-en-21-yl acetate |
| 2-[(8S,9S,10R,13S,14S,17R)-17-Hydroxy-10,13-dimethyl-3,11-dioxo-2,3,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]-2-oxoethylacetat |
| Cortisone Acetate |
| 4-Pregnene-3,11,20-trione, 17α,21-dihydroxy-, 21-acetate |
| Pregn-4-ene-3,11,20-trione, 21-(acetyloxy)-17-hydroxy- |
| Cortisone (acetate) |

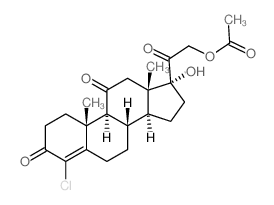 CAS#:28444-83-9
CAS#:28444-83-9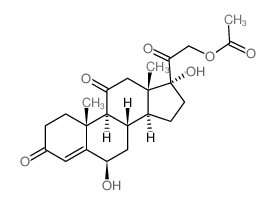 CAS#:13096-52-1
CAS#:13096-52-1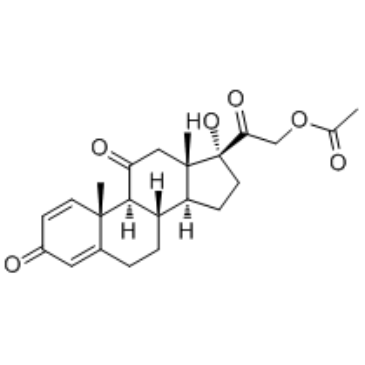 CAS#:125-10-0
CAS#:125-10-0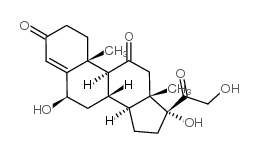 CAS#:16355-28-5
CAS#:16355-28-5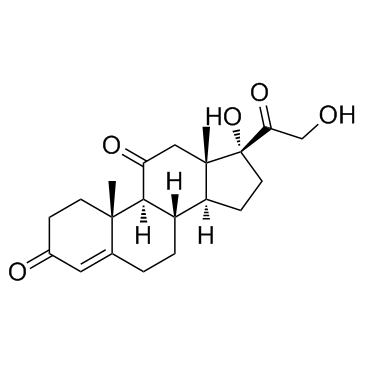 CAS#:53-06-5
CAS#:53-06-5 CAS#:53-03-2
CAS#:53-03-2
