Hydrocortisone

Hydrocortisone structure
|
Common Name | Hydrocortisone | ||
|---|---|---|---|---|
| CAS Number | 50-23-7 | Molecular Weight | 362.460 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 566.5±50.0 °C at 760 mmHg | |
| Molecular Formula | C21H30O5 | Melting Point | 211-214 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 310.4±26.6 °C | |
| Symbol |

GHS08 |
Signal Word | Warning | |
Use of HydrocortisoneHydrocortisone is a steroid hormone or glucocorticoid secreted by the adrenal cortex. |
| Name | cortisol |
|---|---|
| Synonym | More Synonyms |
| Description | Hydrocortisone is a steroid hormone or glucocorticoid secreted by the adrenal cortex. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Hydrocortisone (50 nM) shows a dose-dependent down-regulation of GR transcript in hCMEC/D3 cells. Hydrocortisone supplementation of the serum-reduced cell differentiation medium leads to a significant increase in TER across the hCMEC/D3 monolayer[1]. Hydrocortisone-treated Dendritic cells (DCs) show a decreased expression of MHC II molecules, the costimulatory molecule CD86, and the DC-specific marker CD83, as well as a strongly reduced IL-12 secretion. Hydrocortisone-treated DCs inhibit production of IFN-γ but induce an increased release of IL-4 and no change in IL-5[2]. Hydrocortisone reduces postischemic oxidative stress, perfusion pressure, and transudate formation. Hydrocortisone inhibits postischemic shedding of syndecan-1, heparan sulfate, and hyaluronan as is release of histamine from resident mast cells[3]. |
| Cell Assay | Cells are plated on top of collagen IV-coated transwell chambers for six-well plates (24 mm diameter, membrane material: polyethylene terephthalate (PET), 0.4 μm pores, pore density 1.6×106 cm2) at densities of 2.5×104 cells cm2 per well. When they have reached confluence at day 5, the different experimental sets of cells are transferred to differentiation medium containing reduced amounts of FCS and treated with TNFα or hydrocortisone as indicated. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 566.5±50.0 °C at 760 mmHg |
| Melting Point | 211-214 °C(lit.) |
| Molecular Formula | C21H30O5 |
| Molecular Weight | 362.460 |
| Flash Point | 310.4±26.6 °C |
| Exact Mass | 362.209320 |
| PSA | 94.83000 |
| LogP | 1.43 |
| Vapour Pressure | 0.0±3.5 mmHg at 25°C |
| Index of Refraction | 1.595 |
| InChIKey | JYGXADMDTFJGBT-VWUMJDOOSA-N |
| SMILES | CC12CCC(=O)C=C1CCC1C2C(O)CC2(C)C1CCC2(O)C(=O)CO |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H361 |
| Precautionary Statements | P281 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R62;R63 |
| Safety Phrases | S36/37-S45 |
| RIDADR | 2811.0 |
| WGK Germany | 3 |
| RTECS | GM8925000 |
| Hazard Class | 6.1 |
| HS Code | 2937210000 |
| Precursor 10 | |
|---|---|
| DownStream 9 | |
| HS Code | 2937210000 |
|---|
|
Functional consequence of the MET-T1010I polymorphism in breast cancer.
Oncotarget 6(5) , 2604-14, (2015) Major breast cancer predisposition genes, only account for approximately 30% of high-risk breast cancer families and only explain 15% of breast cancer familial relative risk. The HGF growth factor rec... |
|
|
Cell-cell adhesions and cell contractility are upregulated upon desmosome disruption.
PLoS ONE 9(7) , e101824, (2014) Desmosomes are perturbed in a number of disease states - including genetic disorders, autoimmune and bacterial diseases. Here, we report unexpected changes in other cell-cell adhesion structures upon ... |
|
|
MYC is a critical target of FBXW7.
Oncotarget 6(5) , 3292-305, (2015) MYC deregulation is a driver of many human cancers. Altering the balance of MYC protein levels at the level of transcription, protein stability, or turnover is sufficient to transform cells to a tumor... |
| Medicort |
| 11b,17,21-Trihydroxyprogesterone |
| 17a-Hydroxycorticosterone |
| H-Cort |
| (11β)-11,17,21-Trihydroxypregn-4-ene-3,20-dione |
| 11β,17,21-Trihydroxyprogesterone |
| Cleiton |
| Hydrocortisone |
| MFCD00011654 |
| (11β)-11,17,21-Trihydroxy-pregn-4-ene-3,20-dione |
| Hydroxycorticosterone |
| Hydrocort |
| Kendall's compound F |
| (11b)-11,17,21-trihydroxypregn-4-ene-3,20-dione |
| 4-Pregnene-11b,17a,21-triol-3,20-dione |
| 17-hydroxycorticosterone |
| Hydrocorticosterone |
| 11,17,21-trihydroxy-pregn-4-ene-3,20-dione |
| Hytone |
| Alacort |
| Signef |
| (8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-17-(hydroxyacetyl)-10,13-dimethyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one |
| Efcortelin |
| Hydroxycortisone |
| Hycort |
| pregn-4-ene-3,20-dione, 11,17,21-trihydroxy-, (11b)- |
| hidrocortisona |
| Zenoxone |
| hydrocortisonum |
| CCN 90306A |
| Pregn-4-ene-3,20-dione, 11β,17,21-trihydroxy- |
| HVB |
| Hydrocortal |
| wycort |
| Pregn-4-ene-3,20-dione, 11,17,21-trihydroxy-, (11β)- |
| Optef |
| Hydroskin |
| Eye-Cort |
| EINECS 200-020-1 |
| 17α-Hydroxycorticosterone |
| 11β,17α,21-Trihydroxypregn-4-ene-3,20-dione |
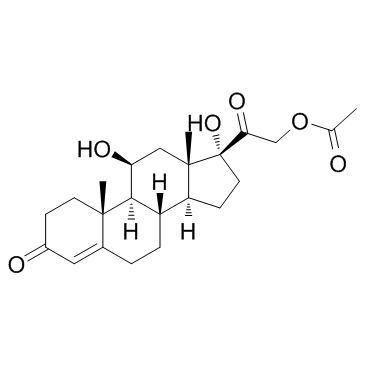 CAS#:50-03-3
CAS#:50-03-3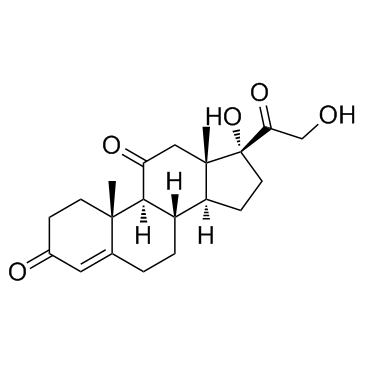 CAS#:53-06-5
CAS#:53-06-5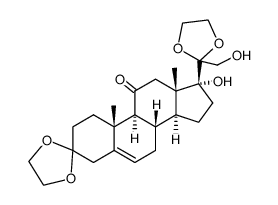 CAS#:101524-47-4
CAS#:101524-47-4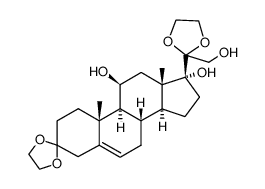 CAS#:76338-54-0
CAS#:76338-54-0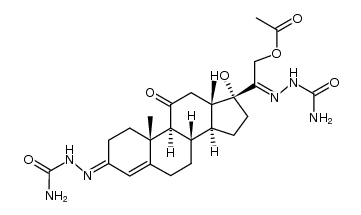 CAS#:104117-71-7
CAS#:104117-71-7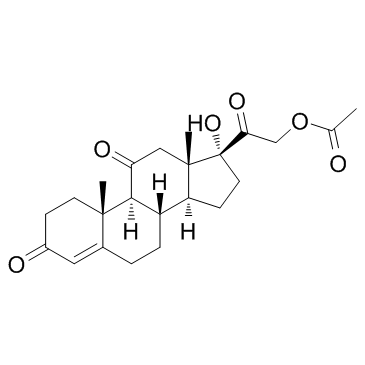 CAS#:50-04-4
CAS#:50-04-4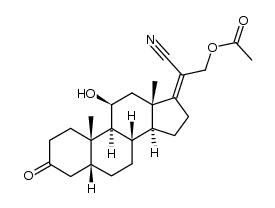 CAS#:102213-14-9
CAS#:102213-14-9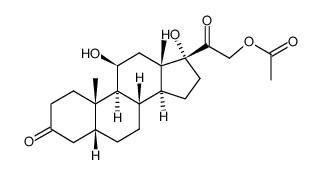 CAS#:64313-94-6
CAS#:64313-94-6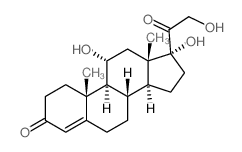 CAS#:566-35-8
CAS#:566-35-8 CAS#:102030-55-7
CAS#:102030-55-7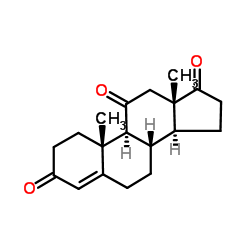 CAS#:382-45-6
CAS#:382-45-6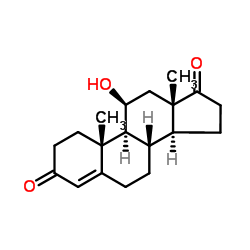 CAS#:382-44-5
CAS#:382-44-5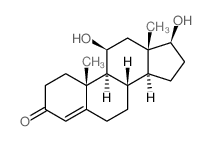 CAS#:1816-85-9
CAS#:1816-85-9 CAS#:2899-95-8
CAS#:2899-95-8 CAS#:1719-79-5
CAS#:1719-79-5 CAS#:3597-45-3
CAS#:3597-45-3 CAS#:50-22-6
CAS#:50-22-6 CAS#:2398-99-4
CAS#:2398-99-4
