O-Desmethylnaproxen

O-Desmethylnaproxen structure
|
Common Name | O-Desmethylnaproxen | ||
|---|---|---|---|---|
| CAS Number | 52079-10-4 | Molecular Weight | 216.23300 | |
| Density | N/A | Boiling Point | 444.1ºC at 760mmHg | |
| Molecular Formula | C13H12O3 | Melting Point | 182-183ºC | |
| MSDS | Chinese USA | Flash Point | 236.5ºC | |
| Symbol |


GHS07, GHS09 |
Signal Word | Warning | |
| Name | O-Desmethylnaproxen |
|---|---|
| Synonym | More Synonyms |
| Boiling Point | 444.1ºC at 760mmHg |
|---|---|
| Melting Point | 182-183ºC |
| Molecular Formula | C13H12O3 |
| Molecular Weight | 216.23300 |
| Flash Point | 236.5ºC |
| Exact Mass | 216.07900 |
| PSA | 57.53000 |
| LogP | 2.73350 |
| Appearance of Characters | white |
| Storage condition | 2-8°C |
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H317-H400 |
| Precautionary Statements | P273-P280 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn,N |
| Risk Phrases | 22-43-50/53 |
| Safety Phrases | 36/37-60-61 |
| RIDADR | UN 3077 9/PG 3 |
| WGK Germany | 3 |
| HS Code | 2918199090 |
|
~98% 
O-Desmethylnaproxen CAS#:52079-10-4 |
| Literature: Auspex Pharmaceuticals, Inc. Patent: US2007/276042 A1, 2007 ; Location in patent: Page/Page column 58 ; US 20070276042 A1 |
|
~83% 
O-Desmethylnaproxen CAS#:52079-10-4 |
| Literature: BEZWADA BIOMEDICAL, LLC. Patent: US2010/209469 A1, 2010 ; |
|
~95% 
O-Desmethylnaproxen CAS#:52079-10-4 |
| Literature: Ferrayoli, Carlos G.; Palacios, Sara M.; Alonso, Ruben A. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1995 , # 12 p. 1635 - 1638 |
|
~% 
O-Desmethylnaproxen CAS#:52079-10-4 |
| Literature: Harrison; Lewis; Nelson; Rooks; Roszkowski; Tomolonis; Fried Journal of medicinal chemistry, 1970 , vol. 13, # 2 p. 203 - 205 |
| HS Code | 2918199090 |
|---|---|
| Summary | 2918199090 other carboxylic acids with alcohol function but without other oxygen function, their anhydrides, halides, peroxides, peroxyacids and their derivatives。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
|
The pharmacokinetic profile of naproxen suppository in man.
Int. J. Clin. Pharmacol. Ther. Toxicol. 26(4) , 190-3, (1988) After a rectal dose of 500 mg in a suppository, naproxen is 6-O-demethylated (20%) and glucuronidated (40%), the metabolites are subsequently excreted renally. The elimination half-life is 15.2 +/- 2.... |
|
|
Sulphation of o-desmethylnaproxen and related compounds by human cytosolic sulfotransferases.
Br. J. Clin. Pharmacol. 60(6) , 632-40, (2005) Naproxen is a nonsteroidal anti-inflammatory drug widely used as an analgesic and anti-inflammatory agent. The conjugated forms of naproxen and O-DMN, its demethylated metabolite, account for 66-92% o... |
|
|
High-performance liquid chromatographic determination of 6-desmethylnaproxen sulfate in human plasma.
J. Chromatogr. A. 383(2) , 449-55, (1986)
|
| (2S)-2-(6-hydroxynaphthalen-2-yl)propanoic acid |
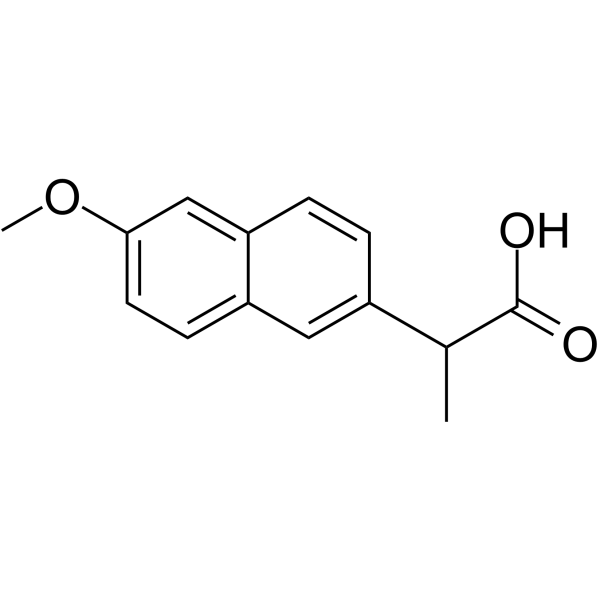
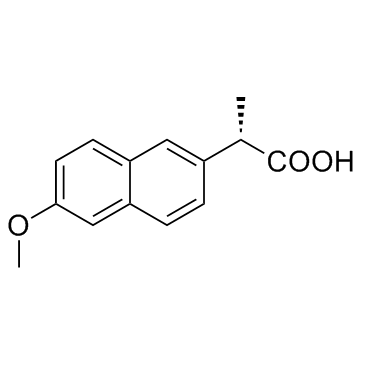


 CAS#:26159-35-3
CAS#:26159-35-3