BML-190

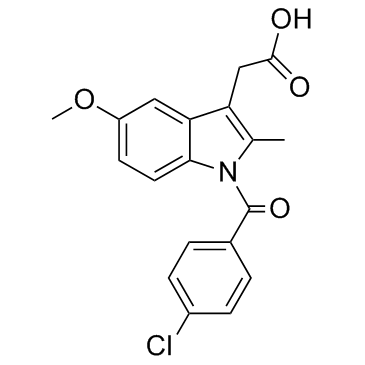
BML-190 structure
|
Common Name | BML-190 | ||
|---|---|---|---|---|
| CAS Number | 2854-32-2 | Molecular Weight | 426.893 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 586.7±50.0 °C at 760 mmHg | |
| Molecular Formula | C23H23ClN2O4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 308.6±30.1 °C | |
Use of BML-190BML-190(IMMA) is a potent and selective CB2 receptor ligand (Ki values are 435 nM and > 2 μM for CB2 and CB1 respectively). IC50 Value: 435 nM(Ki CB2)Target:CB2 receptorin vitro: BML-190 increases the accumulation of cAMP, via forskolin-stimulated mechanism in HEK-293 cells. Alternate studies suggest that BML-190 reduces the toxicity of culture supernatants to SH-SY5Y human neutroblastoma cells. Various research suggests that BML-190 is an essential tool in studying the proliferation of neuroblastoma. BML-190 diminishes LPS-induced NO and IL-6 production in a concentration-dependent manner. BML-190 also inhibits LPS-induced PGE2 production and COX-2 induction. in vivo: |
| Name | 2-[1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl]-1-morpholin-4-ylethanone |
|---|---|
| Synonym | More Synonyms |
| Description | BML-190(IMMA) is a potent and selective CB2 receptor ligand (Ki values are 435 nM and > 2 μM for CB2 and CB1 respectively). IC50 Value: 435 nM(Ki CB2)Target:CB2 receptorin vitro: BML-190 increases the accumulation of cAMP, via forskolin-stimulated mechanism in HEK-293 cells. Alternate studies suggest that BML-190 reduces the toxicity of culture supernatants to SH-SY5Y human neutroblastoma cells. Various research suggests that BML-190 is an essential tool in studying the proliferation of neuroblastoma. BML-190 diminishes LPS-induced NO and IL-6 production in a concentration-dependent manner. BML-190 also inhibits LPS-induced PGE2 production and COX-2 induction. in vivo: |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 586.7±50.0 °C at 760 mmHg |
| Molecular Formula | C23H23ClN2O4 |
| Molecular Weight | 426.893 |
| Flash Point | 308.6±30.1 °C |
| Exact Mass | 426.134644 |
| PSA | 60.77000 |
| LogP | 2.99 |
| Appearance of Characters | solid | off-white |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.625 |
| InChIKey | BJSDNVVWJYDOLK-UHFFFAOYSA-N |
| SMILES | COc1ccc2c(c1)c(CC(=O)N1CCOCC1)c(C)n2C(=O)c1ccc(Cl)cc1 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: >20mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| WGK Germany | 3 |
|---|---|
| HS Code | 2934999090 |
|
~62% 
BML-190 CAS#:2854-32-2 |
| Literature: Kalgutkar, Amit S.; Marnett, Alan B.; Crews, Brenda C.; Remmel, Rory P.; Marnett, Lawrence J. Journal of Medicinal Chemistry, 2000 , vol. 43, # 15 p. 2860 - 2870 |
|
~% 
BML-190 CAS#:2854-32-2 |
| Literature: Gallant, Michel; Dufresne, Claude; Gareau, Yves; Guay, Daniel; Leblanc, Yves; Prasit, Petpiboon; Rochette, Chantal; Sawyer, Nicole; Slipetz, Deborah M.; Tremblay, Nathalie; Metters, Kathleen M.; Labelle, Marc Bioorganic and Medicinal Chemistry Letters, 1996 , vol. 6, # 19 p. 2263 - 2268 |
|
~% 
BML-190 CAS#:2854-32-2 |
| Literature: Gallant, Michel; Dufresne, Claude; Gareau, Yves; Guay, Daniel; Leblanc, Yves; Prasit, Petpiboon; Rochette, Chantal; Sawyer, Nicole; Slipetz, Deborah M.; Tremblay, Nathalie; Metters, Kathleen M.; Labelle, Marc Bioorganic and Medicinal Chemistry Letters, 1996 , vol. 6, # 19 p. 2263 - 2268 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| 2-(1-(4-Chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl)-1-morpholinoethanone |
| Indomethacin morpholinamide |
| Lopac-I-151 |
| BML-190 |
| 2-[1-(4-Chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl]-1-(morpholin-4-yl)ethanone |
| Ethanone (2-[1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl]-1-(4-morpholinyl) |
| Tocris-1383 |
| 2-Methyl-5-methoxy-3-indolyl-essigsaeure-amid |
| IMMA |
| Indomethacin morpholinylamide |
| Ethanone, 2-[1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl]-1-(4-morpholinyl)- |
| 2-[1-(4-Chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl]-1-(4-morpholinyl)ethanone |