Salinomycin
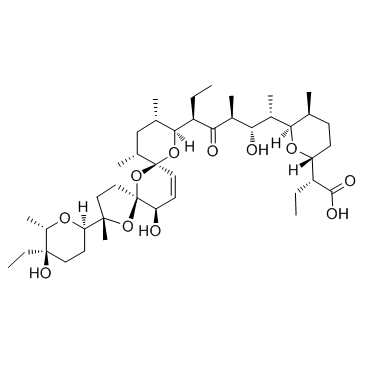
Salinomycin structure
|
Common Name | Salinomycin | ||
|---|---|---|---|---|
| CAS Number | 53003-10-4 | Molecular Weight | 751.00 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 839.2±65.0 °C at 760 mmHg | |
| Molecular Formula | C42H70O11 | Melting Point | 112.5-113.5 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 243.2±27.8 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of SalinomycinSalinomycin is an anticoccidial drug with potent anti-bacterial activity and an novel anticancer agent targeting human cancer stem cells. |
| Name | Salinomycin |
|---|---|
| Synonym | More Synonyms |
| Description | Salinomycin is an anticoccidial drug with potent anti-bacterial activity and an novel anticancer agent targeting human cancer stem cells. |
|---|---|
| Related Catalog | |
| Target |
IC50: 163 nM (Wnt1-stimulated reporter)[1] |
| In Vitro | Salinomycin is a potent inhibitor of the Wnt signaling cascade. Incubation of the malignant lymphocytes with Salinomycin induces apoptosis within 48 h, with a mean IC50 of 230 nM. Salinomycin is also an antibiotic potassium ionophore, has been reported recently to act as a selective breast cancer stem cell inhibitor[1]. Salinomycin is a novel and an effective anticancer drug, inhibits SW620 cells and Cisp-resistant SW620 cells with IC50 of 1.54±0.23 μM and 0.32±0.05 μM, respectively. Salinomycin is found to have the ability to kill both cancer stem cells (CSCs) and therapy-resistant cancer cells. After continuous Salinomycin treatment for 48 h, the apoptotic cells are observed under the microscope and counted randomly at least 100 cells in one field. The number of apoptotic cells which are stained by Hoechst33342 is significantly increased in Cisp-resistant SW620 cells (20.20±3.72) than that of SW620 cells (9.40±2.07) per 100 cells (p<0.05). After treatment with Salinomycin for 48 h, flow cytometric analysis is used to detect the cell apoptosis both in SW620 cells and Cisp-resistant SW620 cells. The cell apoptotic rate in Cisp-resistant SW620 cells (37.82±3.63%) is significantly higher than that of SW620 cells (16.78±2.56%) (p<0.05)[2]. |
| In Vivo | After administration of 4 mg/kg Salinomycin (Sal), 8 mg/kg Salinomycin and 10 uL/g saline water for 6 weeks, the mice are sacrificed. The size of the liver tumors in the Salinomycin treatment groups diminishes compare with the control group. The mean diameter of the tumors decreases from 12.17 mm to 3.67 mm (p<0.05) and the mean volume (V=length×width2×0.5) of the tumors decreases from 819 mm3 to 25.25 mm3 (p<0.05). Next, the tumors are harvested, followed by HE staining, immunohistochemistry, and TUNEL assays, to assess the anti-tumor activity of Salinomycin. HE staining shows that the structure of the liver cancer tissue:nuclei of different sizes, hepatic cord structure is destroyed. Immunohistochemistry shows that PCNA expression is lower after Salinomycin treatment. HE staining and TUNEL assays indicates the Salinomycin-treated groups has higher apoptosis rates than control. Furthermore, immunohistochemistry shows an increased Bax/Bcl-2 ratio after Salinomycin treatment. The protein expression of β-catenin decreases in the Salinomycin treatment groups compared with control[3]. Salinomycin is a kind of monocarboxylic acid polyether type antibiotics, produced by the fermentation of Streptomyces albus, possesses a specific cyclic structure, and can form a complex compound with the pathogenic microorganisms and the extracellular cations of coccidian, especially K+, Na+, Rb+, to alter the intracellular and extracellular ion concentrations[4]. |
| Cell Assay | For cisplatin or Salinomycin IC50 analysis in SW620 cells or Cisp-resistant SW620 cells, cells (1×104/well) are cultured in 96-well plates and treated with different chemotherapeutics (cisplatin, Salinomycin) in different concentrations for 48 h. Then 20 μL of cell counting kit-8 (CCK-8) is added into each of the 96-wells. After 4 h incubation at 37°C, the optical density (OD) values are detected at 450 nm using the scan reader. Cell growth inhibiting rates are described as cell inhibiting curves and the IC50 parameters (inhibiting concentration of 50% cells) are evaluated by Xlfit 5.2 software. For cell proliferation analysis, SW620 cells or Cisp-resistant SW620 cells (5×103/well) are also seeded in 96-well plates in serum-containing medium and treated with cisplatin (5 μM, according to the calculated IC50 values of cisplatin in SW620 cells) for 0, 12, 24, 48, 72 and 96 h. Then 20 μL cell counting kit-8 is added into each of the 96-wells. After 4-h incubation at 37°C, the coloring reactions are also quantified at 450 nm[2]. |
| Animal Admin | Mice[3] Nude mice (nu/nu; 4-6 weeks of age) are used. HepG2 cells are suspended in 100 mL 1:1 serum-free DMEM and Matrigel. Mice are anesthetized with ketamine/xylazine and after surgically opening the abdomen, HepG2 cells are inoculated into the liver parenchyma and mice are monitored every 3 days for 35 days. Finally, 18 nude mice are divided into three groups that are intraperitoneally injected daily for 6 weeks: two Salinomycin-treated groups (4 mg/kg Salinomycin group, 8 mg/kg Salinomycin group) and the control group (saline water group). Rats[4] A total of 10 male rats are used in the experiment. After a routine anesthesia, the abdomen is opened. After a resuspension of high glucose medium not containing serum DMEM, and matrigel, the bladder transitional cancer cell line T24 is inoculated in the parenchyma of bladder in rats, and then the abdomen is sutured. After operation, the rats are randomized into the experiment group and the control group with five in each group. After operation, the rats in the experiment group are immediately given intraperitoneal injection of Salinomycin with a dosage of 8 mg/kg, while the rats in the control group are given intraperitoneal injection of normal saline. A close observation is paid during the drug administration period. After 15 d, the rats are sacrificed by cervical dislocation, and the complete tumor tissues are stripped to observe the tumor growth and metastasis. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 839.2±65.0 °C at 760 mmHg |
| Melting Point | 112.5-113.5 °C(lit.) |
| Molecular Formula | C42H70O11 |
| Molecular Weight | 751.00 |
| Flash Point | 243.2±27.8 °C |
| PSA | 164.04000 |
| LogP | 6.10 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.547 |
| InChIKey | KQXDHUJYNAXLNZ-SDCJTGBBSA-N |
| SMILES | CCC(C(=O)O)C1CCC(C)C(C(C)C(O)C(C)C(=O)C(CC)C2OC3(C=CC(O)C4(CCC(C)(C5CCC(O)(CC)C(C)O5)O4)O3)C(C)CC2C)O1 |
| Storage condition | 2-8°C |
| Stability | Stable, but may be heat sensitive - keep cool. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300 |
| Precautionary Statements | P264-P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic; |
| Risk Phrases | R25 |
| Safety Phrases | S45 |
| RIDADR | UN 3462 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | VO8620000 |
| Packaging Group | II |
| Hazard Class | 6.1(a) |
| HS Code | 2309901000 |
| HS Code | 2309901000 |
|---|
|
Triggering of erythrocyte cell membrane scrambling by salinomycin.
Basic Clin Pharmacol Toxicol. 115(5) , 396-402, (2015) Salinomycin, a polyether ionophore antibiotic effective against a variety of pathogens, has been shown to trigger apoptosis of cancer cells and cancer stem cells. The substance is thus considered for ... |
|
|
High sensitive multiresidue analysis of pharmaceuticals and antifungals in surface water using U-HPLC-Q-Exactive Orbitrap HRMS. Application to the Danube river basin on the Romanian territory.
Sci. Total Environ. 532 , 501-11, (2015) The occurrence of 67 pharmaceutical and antifungal residues in the Danube river on the Romanian territory was studied by using solid-phase extraction (SPE) and LC-Q Exactive Orbitrap high resolution M... |
|
|
ROS-p53-cyclophilin-D signaling mediates salinomycin-induced glioma cell necrosis.
J. Exp. Clin. Cancer Res 34 , 57, (2015) The primary glioblastoma multiforme (GBM) is the most malignant form of astrocytic tumor with an average survival of approximately 12-14 months. The search for novel and more efficient chemo-agents ag... |
| Coxistac |
| 2H-pyran-2-acetic acid, α-ethyl-6-[(1S,2S,3S,5R)-5-[(2S,5S,7R,9S,10S,12R,15R)-2-[(2R,5R,6S)-5-ethyltetrahydro-5-hydroxy-6-methyl-2H-pyran-2-yl]-15-hydroxy-2,10,12-trimethyl-1,6,8-trioxadispiro[4.1.5.3]pentadec-13-en-9-yl]-2-hydroxy-1,3-dimethyl-4-oxoheptyl]tetrahydro-5-methyl-, (αR,2R,5S,6R)- |
| Antibiotic 61477 |
| (2R)-2-{(2R,5S,6R)-6-[(2S,3S,4S,6R)-6-{(2S,5S,7R,9S,10S,12R,15R)-2-[(2R,5R,6S)-5-Ethyl-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]-15-hydroxy-2,10,12-trimethyl-1,6,8-trioxadispiro[4.1.5.3]pentadec-13-en-9-yl}-3-hydroxy-4-methyl-5-oxo-2-octanyl]-5-methyltetrahydro-2H-pyran-2-yl}butanoic acid |
| salinomycin acid |
| MFCD00036900 |
| SALINOMYCIN SODIUM |
| Salinomycin |
| 2H-Pyran-2-acetic acid, α-ethyl-6-[(1S,2S,3S,5R)-5-[(2S,5S,7R,9S,10S,12R,15R)-2-[(2R,5R,6S)-5-ethyltetrahydro-5-hydroxy-6-methyl-2H-pyran-2-yl]-15-hydroxy-2,10,12-trimethyl-1,6,8-trioxadispiro[4.1 .5.3]pentadec-13-en-9-yl]-2-hydroxy-1,3-dimethyl-4-oxoheptyl]tetrahydro-5-methyl-, (αR,2R,5S,6R)- |
| EINECS 258-290-1 |
| SALINOMYCIN GRANULE |
| SALINOMYCIN SODIUM SALT |
| Bio-cox |
| (2R)-2-{(2R,5S,6R)-6-[(2S,3S,4S,6R)-6-{(2S,5S,7R,9S,10S,12R,15R)-2-[(2R,5R,6S)-5-Ethyl-5-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]-15-hydroxy-2,10,12-trimethyl-1,6,8-trioxadispiro[4.1.5.3]pentadec-13-en-9-yl}-3-hydroxy-4-methyl-5-oxooctan-2-yl]-5-methyltetrahydro-2H-pyran-2-yl}butanoic acid |
 CAS#:55721-31-8
CAS#:55721-31-8 CAS#:120330-27-0
CAS#:120330-27-0 CAS#:2280-44-6
CAS#:2280-44-6 CAS#:81496-83-5
CAS#:81496-83-5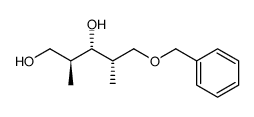 CAS#:81520-10-7
CAS#:81520-10-7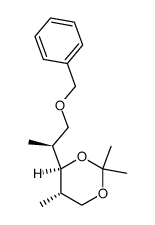 CAS#:81470-77-1
CAS#:81470-77-1![(S)-2-[(2R,3S,6R)-6-[(S)-1-(benzyloxymethyl)propyl]-3-methyltetrahydropyran-2-yl]propan-1-ol Structure](https://image.chemsrc.com/caspic/344/107207-23-8.png) CAS#:107207-23-8
CAS#:107207-23-8