CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
AY5950000
-
CHEMICAL NAME :
-
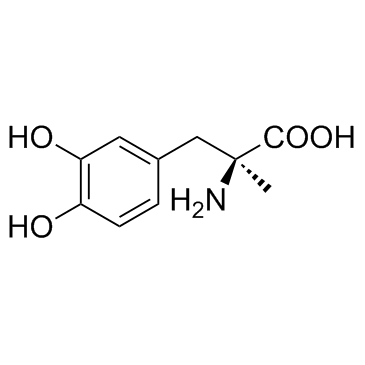
Alanine, 3-(3,4-dihydroxyphenyl)-2-methyl-, L-(-)-
-
CAS REGISTRY NUMBER :
-
555-30-6
-
LAST UPDATED :
-
199707
-
DATA ITEMS CITED :
-
32
-
MOLECULAR FORMULA :
-
C10-H13-N-O4
-
MOLECULAR WEIGHT :
-
211.24
-
WISWESSER LINE NOTATION :
-
QVXZ1&1R CQ DQ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
900 mg/kg/13W-I
-
TOXIC EFFECTS :
-
Behavioral - tremor
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
1830 mg/kg/17W-I
-
TOXIC EFFECTS :
-
Peripheral Nerve and Sensation - fasciculations Behavioral - hallucinations, distorted perceptions Behavioral - tremor
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
1071 mg/kg/22W-I
-
TOXIC EFFECTS :
-
Skin and Appendages - dermatitis, allergic (after systemic exposure)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
44 gm/kg/3Y-I
-
TOXIC EFFECTS :
-
Gastrointestinal - necrotic changes
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
300 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
5300 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
150 mg/kg
-
TOXIC EFFECTS :
-
Cardiac - pulse rate increase, without fall in BP Vascular - BP lowering not characterized in autonomic section
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1700 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
713 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
713 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
56875 mg/kg/13W-C
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis) Blood - changes in bone marrow (not otherwise specified) Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
136 gm/kg/13W-C
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis) Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
12105 mg/kg/50D-I
-
TOXIC EFFECTS :
-
Endocrine - changes in spleen weight Kidney, Ureter, Bladder - changes in bladder weight Blood - changes in leukocyte (WBC) count
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
10950 mg/kg
-
SEX/DURATION :
-
female 2 year(s) pre-mating female 1-12 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders Reproductive - Maternal Effects - breasts, lactation (prior to or during pregnancy)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
1680 mg/kg
-
SEX/DURATION :
-
female 28-36 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - other neonatal measures or effects Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain) Reproductive - Effects on Newborn - biochemical and metabolic
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
2306 mg/kg
-
SEX/DURATION :
-
female 5-14 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2100 mg/kg
-
SEX/DURATION :
-
female 1-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
7500 mg/kg
-
SEX/DURATION :
-
female 6-20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - parturition Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
7500 mg/kg
-
SEX/DURATION :
-
female 6-20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
13 gm/kg
-
SEX/DURATION :
-
male 65 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count) Reproductive - Paternal Effects - testes, epididymis, sperm duct Reproductive - Fertility - male fertility index (e.g. # males impregnating females per # males exposed to fertile nonpregnant females)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
300 mg/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
480 mg/kg
-
SEX/DURATION :
-
male 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
960 mg/kg
-
SEX/DURATION :
-
male 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
80 mg/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
150 mg/kg
-
SEX/DURATION :
-
male 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - other effects on male
-
TYPE OF TEST :
-
Dominant lethal test
MUTATION DATA
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TEST SYSTEM :
-
Rodent - hamster Lung
-
DOSE/DURATION :
-
37 mg/L
-
REFERENCE :
-
GMCRDC Gann Monograph on Cancer Research. (Plenum Pub. Corp., 233 Spring St., New York, NY 10013) No. 11- 1971- Volume(issue)/page/year: 27,95,1981 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X3201 No. of Facilities: 366 (estimated) No. of Industries: 2 No. of Occupations: 13 No. of Employees: 16478 (estimated) No. of Female Employees: 11629 (estimated)
|
 CAS#:21852-32-4
CAS#:21852-32-4 CAS#:5846-22-0
CAS#:5846-22-0 CAS#:33859-00-6
CAS#:33859-00-6![(3S,4R)-1-benzyl-3-[2-oxo-4(S)-phenyloxazolidinyl]-4-(3,4-dimethoxyphenyl)azetidin-2-one Structure](https://image.chemsrc.com/caspic/496/111793-95-4.png) CAS#:111793-95-4
CAS#:111793-95-4![(3S,4R)-1-benzyl-3-methyl-3-[2-oxo-(4S)-phenyloxazolidinyl]-4-(3,4-dimethoxyphenyl)azetidin-2-one Structure](https://image.chemsrc.com/caspic/458/111793-96-5.png) CAS#:111793-96-5
CAS#:111793-96-5 CAS#:39948-18-0
CAS#:39948-18-0![methyl-2 [(tri-O-benzoyl-2',3',5' β-D-ribofuranosyl)-3 hydroxy-4 phenyl]-3 alanine structure](https://image.chemsrc.com/caspic/029/79439-78-4.png) CAS#:79439-78-4
CAS#:79439-78-4 CAS#:14215-97-5
CAS#:14215-97-5 CAS#:4821-01-6
CAS#:4821-01-6