Magnesium stearate
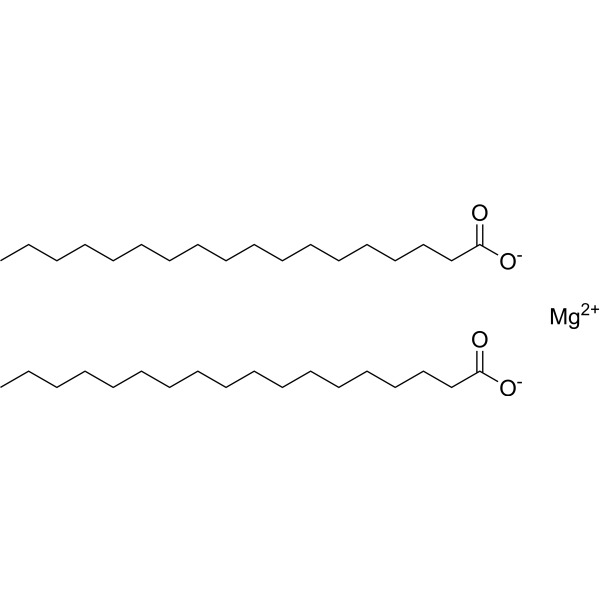
Magnesium stearate structure
|
Common Name | Magnesium stearate | ||
|---|---|---|---|---|
| CAS Number | 557-04-0 | Molecular Weight | 591.24 | |
| Density | 1.028g/cm3 | Boiling Point | 359.4ºC at 760mmHg | |
| Molecular Formula | C36H70MgO4 | Melting Point | 200 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 162.4ºC | |
Use of Magnesium stearateMagnesium stearate is a commonly used pharmaceutical lubricant. But Magnesium stearate leads to an adverse effect on bonding between particles. Magnesium stearate can be used as an excipient, such as lubricant. Pharmaceutical excipients, or pharmaceutical auxiliaries, refer to other chemical substances used in the pharmaceutical process other than pharmaceutical ingredients. Pharmaceutical excipients generally refer to inactive ingredients in pharmaceutical preparations, which can improve the stability, solubility and processability of pharmaceutical preparations. Pharmaceutical excipients also affect the absorption, distribution, metabolism, and elimination (ADME) processes of co-administered drugs[1][2]. |
| Name | magnesium distearate |
|---|---|
| Synonym | More Synonyms |
| Description | Magnesium stearate is a commonly used pharmaceutical lubricant. But Magnesium stearate leads to an adverse effect on bonding between particles. Magnesium stearate can be used as an excipient, such as lubricant. Pharmaceutical excipients, or pharmaceutical auxiliaries, refer to other chemical substances used in the pharmaceutical process other than pharmaceutical ingredients. Pharmaceutical excipients generally refer to inactive ingredients in pharmaceutical preparations, which can improve the stability, solubility and processability of pharmaceutical preparations. Pharmaceutical excipients also affect the absorption, distribution, metabolism, and elimination (ADME) processes of co-administered drugs[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.028g/cm3 |
|---|---|
| Boiling Point | 359.4ºC at 760mmHg |
| Melting Point | 200 °C(lit.) |
| Molecular Formula | C36H70MgO4 |
| Molecular Weight | 591.24 |
| Flash Point | 162.4ºC |
| Exact Mass | 590.512451 |
| PSA | 52.60000 |
| LogP | 12.50830 |
| Index of Refraction | 1.45 (25ºC) |
| Storage condition | Store at RT. |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| Water Solubility | Insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | - |
| RTECS | WI4390000 |
| HS Code | 29157030 |
|
~% 
Magnesium stearate CAS#:557-04-0 |
| Literature: US2014/100236 A1, ; Page/Page column ; |
| Precursor 1 | |
|---|---|
| DownStream 2 | |
| HS Code | 29157030 |
|---|
|
The stress stability of olanzapine: studies of interactions with excipients in solid state pharmaceutical formulations.
Drug Dev. Ind. Pharm. 41(3) , 502-14, (2015) Stress stability testing represents an important part of the drug development process. It is used as an important tool for the identification of degradation products and degradation pathways, as well ... |
|
|
Antisolvent precipitation of novel xylitol-additive crystals to engineer tablets with improved pharmaceutical performance.
Int. J. Pharm. 477(1-2) , 282-93, (2014) The purpose of this work was to develop stable xylitol particles with modified physical properties, improved compactibility and enhanced pharmaceutical performance without altering polymorphic form of... |
|
|
Adrenaline (epinephrine) microcrystal sublingual tablet formulation: enhanced absorption in a preclinical model.
J. Pharm. Pharmacol. 67(1) , 20-5, (2014) For anaphylaxis treatment in community settings, adrenaline (epinephrine) administration using an auto-injector in the thigh is universally recommended. Despite this, many people at risk of anaphylaxi... |
| MAGNESIUM DISTEARATE |
| Magnesium stearate |
| synpro 90 |
| MFCD00036391 |
| Stearic acid, magnesium salt |
| MAGNESIUM STEARATE (E-470B, F.C.C.) ADITIO |
| EINECS 209-150-3 |
| Octadecanoic acid, magnesium salt |
| stearic acid magnesium salt |
| Octadecanoic acid, magnesium salt (2:1) |
| stearatedemagnesium |
| MAGENSIUM STEARATE |
| MAGNESIUMSTEARATE |
| MEGNESIUM STEATATE |
| MAGNESIUMSTEARATEBP |
| Magnesium dioctadecanoate |
| Microcrystalline cellulose use as the oral steroid filler |
| Dibasic magnesium stearate |
| MAGNESIUM STEARATE API |
| magnesiumdistearate |
| Synpro Magnesium Stearate 90 |
| OCTADECANOIC ACID MAGNESIUM SALT |
| stearate magnesium |
| Magnesiumdistearat |
| MAGNESIUM STEARATE NF |
| MAGNESIUM OCTADECANOATE |
| magnesium sterate |
| stearic acid,magnesium stearate |
| Dolomol |
| Magnesiumstearat |
| OCTADECANOICACID, MAGNESIUM SALT (2:1) |
| MAGNESIUMSTEARATE,FCC |
| petracmg20nf |
| MAGNESIUM STEARATE, FCC |
| Magnesium Stearate Bp |
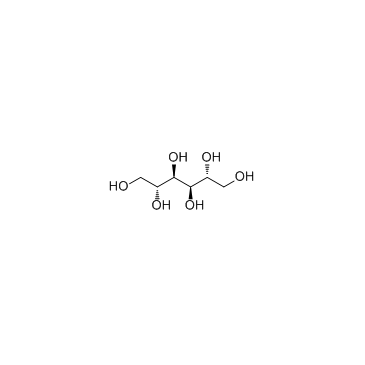
 CAS#:39133-31-8
CAS#:39133-31-8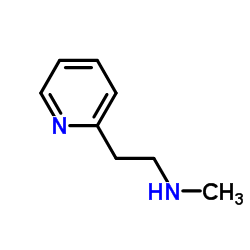 CAS#:5638-76-6
CAS#:5638-76-6
