3alpha,21-dihydroxy-5beta-pregnan-20-one
Modify Date: 2025-08-24 13:02:23
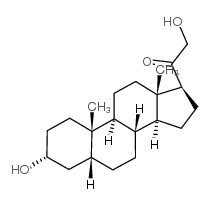
3alpha,21-dihydroxy-5beta-pregnan-20-one structure
|
Common Name | 3alpha,21-dihydroxy-5beta-pregnan-20-one | ||
|---|---|---|---|---|
| CAS Number | 567-03-3 | Molecular Weight | 334.49300 | |
| Density | 1.115g/cm3 | Boiling Point | 470.3ºC at 760mmHg | |
| Molecular Formula | C21H34O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 252.3ºC | |
Use of 3alpha,21-dihydroxy-5beta-pregnan-20-oneTetrahydrodeoxycorticosterone, an neurosteroid, is a potent positive allosteric modulator (PAM) of GABAA receptor. Tetrahydrodeoxycorticosterone has potent neuroinhibitory properties[1][2]. |
| Name | Tetrahydro 11-Deoxycorticosterone |
|---|---|
| Synonym | More Synonyms |
| Description | Tetrahydrodeoxycorticosterone, an neurosteroid, is a potent positive allosteric modulator (PAM) of GABAA receptor. Tetrahydrodeoxycorticosterone has potent neuroinhibitory properties[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | The endogenous neurosteroid Tetrahydrodeoxycorticosterone (THDOC) at physiological concentrations selectively enhances tonic currents mediated by αβδ receptors[1]. In hippocampus, 10 nM Tetrahydrodeoxycorticosterone reduces neuronal excitability by augmenting tonic αβδ receptor currents. In thalamocortical neurons, although 100 nM Tetrahydrodeoxycorticosterone enhances tonic currents, 10 nM Tetrahydrodeoxycorticosterone does not[1]. |
| In Vivo | Concentrations of Tetrahydrodeoxycorticosterone (THDOC) in brain tissue from mice with hepatic encephalopathy (HE) resulting from toxic liver injury are sufficient to induce sedation in animals of the same species[2]. |
| References |
| Density | 1.115g/cm3 |
|---|---|
| Boiling Point | 470.3ºC at 760mmHg |
| Molecular Formula | C21H34O3 |
| Molecular Weight | 334.49300 |
| Flash Point | 252.3ºC |
| Exact Mass | 334.25100 |
| PSA | 57.53000 |
| LogP | 3.56760 |
| Index of Refraction | 1.54 |
| InChIKey | CYKYBWRSLLXBOW-DATPGIFZSA-N |
| SMILES | CC12CCC(O)CC1CCC1C2CCC2(C)C(C(=O)CO)CCC12 |
| tetrahydrodeoxycorticosterone |
 CAS#:1693-62-5
CAS#:1693-62-5 CAS#:2402-24-6
CAS#:2402-24-6 CAS#:64-85-7
CAS#:64-85-7 CAS#:1491-77-6
CAS#:1491-77-6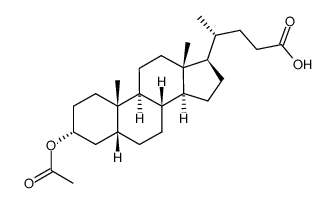 CAS#:4057-84-5
CAS#:4057-84-5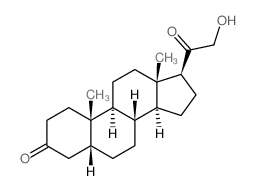 CAS#:303-01-5
CAS#:303-01-5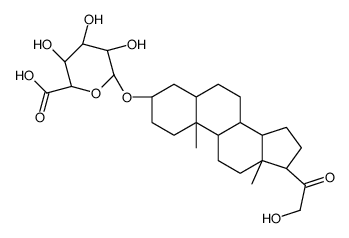 CAS#:56162-36-8
CAS#:56162-36-8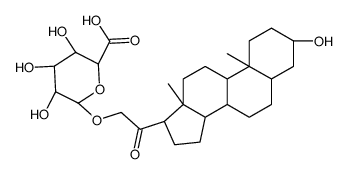 CAS#:56162-37-9
CAS#:56162-37-9