Gabexate mesylate
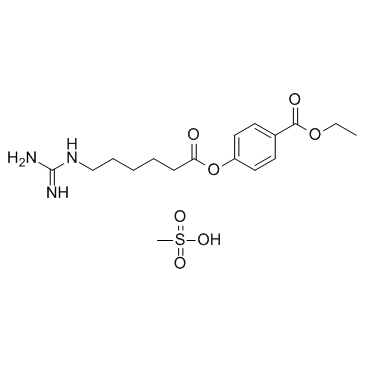
Gabexate mesylate structure
|
Common Name | Gabexate mesylate | ||
|---|---|---|---|---|
| CAS Number | 56974-61-9 | Molecular Weight | 417.477 | |
| Density | N/A | Boiling Point | 508.6ºC at 760 mmHg | |
| Molecular Formula | C17H27N3O7S | Melting Point | 91 °C | |
| MSDS | Chinese | Flash Point | 261.4ºC | |
Use of Gabexate mesylateGabexate Mesylate is a Factor X inhibitor; serine protease inhibitor .Target: Factor XGabexate mesylate is a non-antigenic synthetic inhibitor of trypsin-like serine proteinases that is therapeutically used in the treatment of pancreatitis and disseminated intravascular coagulation and as a regional anticoagulant for hemodialysis. Values of the inhibition constant (K(i)) for gabexate mesylate binding to human and bovine tryptase were 3.4 x 10(-9) M and 1.8 x 10(-7) M (at pH 7.4 and 37.0 degrees ), respectively. Gabexate mesylate inhibited the fibrinogenolytic activity of human tryptase [1]. Gabexate Mesylate decreased the TNFalpha production of LPS-stimulated monocytes as shown by the inhibition of mRNA expression and increased the IL-10 production of LPS-stimulated monocytes. Gabexate Mesylate also suppressed the NFkappaB activity of LPS-stimulated monocytes. Inhibitory effect of Gabexate Mesylate on the TNFalpha production of activated human monocytes is mediated by the suppression of NFkappaB activation [2]. Gabexate mesylate inhibits competitively constitutive and inducible NO synthase (cNOS and iNOS, respectively), with Kivalues of 1.0×10?4M and 5.0×10?3M, respectively, at pH 7.4 and 37.0°C. gabexate mesylate increases iNOS mRNA expression in rat C6 glioma cells, as induced byE. colilipopolysaccharide plus interferon-γ. Gabexate mesylate inhibits dose-dependently nitrite production (i.e. NO release) in rat C6 glioma cells, as induced byE. colilipopolysaccharide plus interferon-γ [3]. |
| Name | Gabexate Mesylate |
|---|---|
| Synonym | More Synonyms |
| Description | Gabexate Mesylate is a Factor X inhibitor; serine protease inhibitor .Target: Factor XGabexate mesylate is a non-antigenic synthetic inhibitor of trypsin-like serine proteinases that is therapeutically used in the treatment of pancreatitis and disseminated intravascular coagulation and as a regional anticoagulant for hemodialysis. Values of the inhibition constant (K(i)) for gabexate mesylate binding to human and bovine tryptase were 3.4 x 10(-9) M and 1.8 x 10(-7) M (at pH 7.4 and 37.0 degrees ), respectively. Gabexate mesylate inhibited the fibrinogenolytic activity of human tryptase [1]. Gabexate Mesylate decreased the TNFalpha production of LPS-stimulated monocytes as shown by the inhibition of mRNA expression and increased the IL-10 production of LPS-stimulated monocytes. Gabexate Mesylate also suppressed the NFkappaB activity of LPS-stimulated monocytes. Inhibitory effect of Gabexate Mesylate on the TNFalpha production of activated human monocytes is mediated by the suppression of NFkappaB activation [2]. Gabexate mesylate inhibits competitively constitutive and inducible NO synthase (cNOS and iNOS, respectively), with Kivalues of 1.0×10?4M and 5.0×10?3M, respectively, at pH 7.4 and 37.0°C. gabexate mesylate increases iNOS mRNA expression in rat C6 glioma cells, as induced byE. colilipopolysaccharide plus interferon-γ. Gabexate mesylate inhibits dose-dependently nitrite production (i.e. NO release) in rat C6 glioma cells, as induced byE. colilipopolysaccharide plus interferon-γ [3]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 508.6ºC at 760 mmHg |
|---|---|
| Melting Point | 91 °C |
| Molecular Formula | C17H27N3O7S |
| Molecular Weight | 417.477 |
| Flash Point | 261.4ºC |
| Exact Mass | 417.156982 |
| PSA | 177.25000 |
| LogP | 3.58790 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn: Harmful; |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | DG2800800 |
|
~76% 
Gabexate mesylate CAS#:56974-61-9 |
| Literature: Isobe, Toshio; Ishikawa, Tsutomu Journal of Organic Chemistry, 1999 , vol. 64, # 19 p. 6984 - 6988 |
|
Involvement of proteinase activated receptor-2 in the vascular response to sphingosine 1-phosphate.
Clin. Sci. 126(8) , 545-56, (2014) S1P (sphingosine 1-phosphate) represents one of the key latest additions to the list of vasoactive substances that modulate vascular tone. PAR-2 (proteinase activated receptor-2) has been shown to be ... |
|
|
Role of TRPV1 in nociception and edema induced by monosodium urate crystals in rats.
Pain 152(8) , 1777-88, (2011) Gout is characterized by the deposition of monosodium urate (MSU) crystals. Despite being one of the most painful forms of arthritis, gout and the mechanisms responsible for its acute attacks are poor... |
|
|
Neonatal amygdala lesions advance pubertal timing in female rhesus macaques.
Psychoneuroendocrinology 51 , 307-17, (2014) Social context influences the timing of puberty in both humans and nonhuman primates, such as delayed first ovulation in low-ranking rhesus macaques, but the brain region(s) mediating the effects of s... |
| benzoic acid, 4-[[6-[(diaminomethylene)amino]-1-oxohexyl]oxy]-, ethyl ester, methanesulfonate (1:1) |
| ethyl 4-({6-[(diaminomethylidene)amino]hexanoyl}oxy)benzoate methanesulfonate (1:1) |
| Ethyl 4-[(6-carbamimidamidohexanoyl)oxy]benzoate methanesulfonate (1:1) |
| Gabexate monomethanesulfonate |
| Benzoic acid, 4-[[6-[(aminoiminomethyl)amino]-1-oxohexyl]oxy]-, ethyl ester, methanesulfonate (1:1) |
| MFCD00210299 |
| Ethyl 4-({6-[(diaminomethylene)amino]hexanoyl}oxy)benzoate methanesulfonate (1:1) |
| Gabexate mesylate |
 CAS#:80693-42-1
CAS#:80693-42-1