CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
WO9275000
-
CHEMICAL NAME :
-
Sulfanilamide, N(sup 1)-(4,6-dimethyl-2-pyrimidinyl)-
-
CAS REGISTRY NUMBER :
-
57-68-1
-
BEILSTEIN REFERENCE NO. :
-
0261304
-
LAST UPDATED :
-
199706
-
DATA ITEMS CITED :
-
19
-
MOLECULAR FORMULA :
-
C12-H14-N4-O2-S
-
MOLECULAR WEIGHT :
-
278.36
-
WISWESSER LINE NOTATION :
-
T6N CNJ BMSWR DZ& D1 F1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
ABMGAJ Acta Biologica et Medica Germanica. (Berlin, Ger. Dem. Rep.) V.1-41, 1958-82. For publisher information, see BBIADT. Volume(issue)/page/year: 27,141,1971
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
50 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: 6,392,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1060 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - dyspnea
-
REFERENCE :
-
AITEAT Archivum Immunologiae et Therapiae Experimentalis. (Ars Polona, POB 1001, 00-068 Warsaw 1, Poland) V.10- 1962- Volume(issue)/page/year: 16,804,1968
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1440 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
ABMGAJ Acta Biologica et Medica Germanica. (Berlin, Ger. Dem. Rep.) V.1-41, 1958-82. For publisher information, see BBIADT. Volume(issue)/page/year: 27,141,1971
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1776 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
HBTXAC "Handbook of Toxicology," 4 vols., Philadelphia, W.B. Saunders Co., 1956-59 Volume(issue)/page/year: 5,163,1959
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
2450 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
DIPHAH Dissertationes Pharmaceuticae. (Warsaw, Poland) V.1-17, 1949-65. For publisher information, see PJPPAA. Volume(issue)/page/year: 11,149,1959 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
52560 mg/kg/2Y-C
-
TOXIC EFFECTS :
-
Endocrine - changes in thyroid weight Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
REFERENCE :
-
JTEHD6 Journal of Toxicology and Environmental Health. (Hemisphere Pub., 1025 Vermont Ave., NW, Washington, DC 20005) V.1- 1975/76- Volume(issue)/page/year: 22,175,1987 ** TUMORIGENIC DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
104 gm/kg/2Y-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Endocrine - thyroid tumors
-
REFERENCE :
-
FCTOD7 Food and Chemical Toxicology. (Pergamon Press Inc., Maxwell House, Fairview Park, Elmsford, NY 10523) V.20- 1982- Volume(issue)/page/year: 28,157,1990
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Implant
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
5000 mg/kg
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Tumorigenic - tumor types after systemic administration not seen spontaneously
-
REFERENCE :
-
ACRAAX Acta Radiologica. (Stockholm, Sweden) V.1-58, 1921-62. For publisher information, see ACRDA8. Volume(issue)/page/year: 37,258,1952
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
419 gm/kg/2Y-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Endocrine - thyroid tumors
-
REFERENCE :
-
FCTOD7 Food and Chemical Toxicology. (Pergamon Press Inc., Maxwell House, Fairview Park, Elmsford, NY 10523) V.20- 1982- Volume(issue)/page/year: 27,455,1989 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
6850 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - urogenital system
-
REFERENCE :
-
NTIS** National Technical Information Service. (Springfield, VA 22161) Formerly U.S. Clearinghouse for Scientific & Technical Information. Volume(issue)/page/year: PB83-151035
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
8650 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
NTIS** National Technical Information Service. (Springfield, VA 22161) Formerly U.S. Clearinghouse for Scientific & Technical Information. Volume(issue)/page/year: PB83-151035
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
46200 mg/kg
-
SEX/DURATION :
-
male 6 week(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - male fertility index (e.g. # males impregnating females per # males exposed to fertile nonpregnant females)
-
REFERENCE :
-
JRPFA4 Journal of Reproduction and Fertility. (Biochemical Soc. Book Depot, POB 32, Commerce Way, Colchester, Essex CO2 8HP, UK) V.1- 1960- Volume(issue)/page/year: 81,259,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
25200 mg/kg
-
SEX/DURATION :
-
female 6-19 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
NTIS** National Technical Information Service. (Springfield, VA 22161) Formerly U.S. Clearinghouse for Scientific & Technical Information. Volume(issue)/page/year: PB85-105690 *** REVIEWS *** TOXICOLOGY REVIEW DPIRDU Dangerous Properties of Industrial Materials Report. (Van Nostrand Reinhold, 115 Fifth Ave., New York, NY 10003) V.1- 1981- Volume(issue)/page/year: 2(2),5,1982 TOXICOLOGY REVIEW FAATDF Fundamental and Applied Toxicology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1981- Volume(issue)/page/year: 13,747,1989 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 83543 No. of Facilities: 1105 (estimated) No. of Industries: 6 No. of Occupations: 12 No. of Employees: 6918 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - 83543 No. of Facilities: 1303 (estimated) No. of Industries: 4 No. of Occupations: 22 No. of Employees: 17376 (estimated) No. of Female Employees: 2625 (estimated)
|
 CAS#:100-90-3
CAS#:100-90-3 CAS#:767-15-7
CAS#:767-15-7 CAS#:121-60-8
CAS#:121-60-8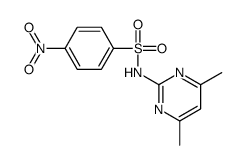 CAS#:153312-38-0
CAS#:153312-38-0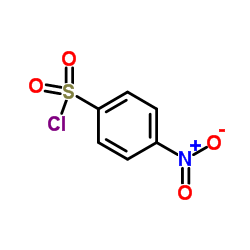 CAS#:98-74-8
CAS#:98-74-8 CAS#:4472-44-0
CAS#:4472-44-0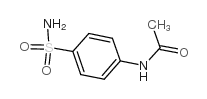 CAS#:121-61-9
CAS#:121-61-9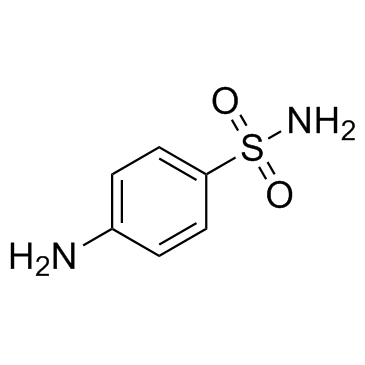 CAS#:63-74-1
CAS#:63-74-1 CAS#:123-54-6
CAS#:123-54-6 CAS#:57-67-0
CAS#:57-67-0 CAS#:2315-08-4
CAS#:2315-08-4 CAS#:108-79-2
CAS#:108-79-2 CAS#:121-57-3
CAS#:121-57-3 CAS#:108-95-2
CAS#:108-95-2![4-[4-[(4,6-dimethylpyrimidin-2-yl)sulfamoyl]anilino]-4-oxobut-2-enoic acid structure](https://image.chemsrc.com/caspic/485/37560-05-7.png) CAS#:37560-05-7
CAS#:37560-05-7![4-[[4-[[(4,6-dimethyl-2-pyrimidinyl)amino]sulphonyl]phenyl]amino]-4-oxobutyric acid structure](https://image.chemsrc.com/caspic/454/85828-79-1.png) CAS#:85828-79-1
CAS#:85828-79-1
