2,2,2-Trichloroacetamide
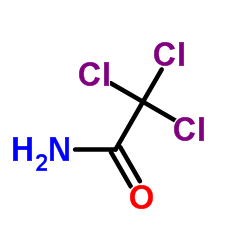
2,2,2-Trichloroacetamide structure
|
Common Name | 2,2,2-Trichloroacetamide | ||
|---|---|---|---|---|
| CAS Number | 594-65-0 | Molecular Weight | 162.402 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 239.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C2H2Cl3NO | Melting Point | 139-141 °C(lit.) | |
| MSDS | USA | Flash Point | 88.5±25.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 2,2,2-Trichloroacetamide |
|---|---|
| Synonym | More Synonyms |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 239.0±0.0 °C at 760 mmHg |
| Melting Point | 139-141 °C(lit.) |
| Molecular Formula | C2H2Cl3NO |
| Molecular Weight | 162.402 |
| Flash Point | 88.5±25.9 °C |
| Exact Mass | 160.920197 |
| PSA | 43.09000 |
| LogP | 1.15 |
| Vapour Pressure | 0.0±0.4 mmHg at 25°C |
| Index of Refraction | 1.521 |
| Stability | Stable. Incompatible with strong acids, strong bases, strong oxidizing agents, strong reducing agents. |
| Water Solubility | 13 g/L (20 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S36/37-S45-S37/39-S26 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | AC9275000 |
| Hazard Class | 6.1 |
| HS Code | 29241900 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
An improved synthesis of (-)-5,11-dideoxytetrodotoxin.
J. Org. Chem. 78(4) , 1699-705, (2013) We describe an improved synthesis of (-)-5,11-dideoxytetrodotoxin from an enone, which was used for synthesis of tetrodotoxin and its analogues in this laboratory. One of the major modifications was t... |
|
|
Activation of glycosyl trichloroacetimidates with perchloric acid on silica (HClO(4)-SiO(2)) provides enhanced alpha-selectivity.
Carbohydr. Res. 345(14) , 2074-8, (2010) Obtaining high stereoselectivity in glycosylation reactions is often challenging in the absence of neighboring group participation. In this study, we demonstrate that activation of glycosyl trichloroa... |
|
|
Synthetic studies toward the anthrax tetrasaccharide: alternative synthesis of this antigen
Carbohydr. Res. 356 , 115-31, (2012) The synthesis of the anthrax tetrasaccharide, amenable for conjugation, has been envisaged by both [2+2] and [1+3] approaches from D-fucose and L-rhamnose. The successful route reported herein relies ... |
| Acetamide, 2,2,2-trichloro- |
| TRICHLOROACETAMIDE |
| 2,2,2-Trichloroacetamide |
| 3,3,3-trichloroacetamide |
| chloraloxime |
| 2,2,13,13-TETRAMETHYL-4,7,11-TRIOXO-3,12-DIOXA-5,8-DIAZATETRADECANE-6-CARBOXYLIC ACID |
| 2,2,2-Chloroacetamide |
| a,a,a-Trichloroacetamide |
| trichloro-acetic acid amide |
| 2,2,2-Trichloroaceta |
| trichloroacetyl amide |
| MFCD00008009 |
| EINECS 209-849-3 |
| 2,2,2-trichloro-acetamid |
| Trichloroacetaldoxime |
| 2,2,2-trichloro-acetamide |
| Trichloroacetimidic acid |
 CAS#:98946-18-0
CAS#:98946-18-0 CAS#:772-33-8
CAS#:772-33-8 CAS#:1067910-79-5
CAS#:1067910-79-5 CAS#:81927-55-1
CAS#:81927-55-1 CAS#:14131-63-6
CAS#:14131-63-6 CAS#:76-02-8
CAS#:76-02-8 CAS#:545-06-2
CAS#:545-06-2 CAS#:624-76-0
CAS#:624-76-0 CAS#:598-99-2
CAS#:598-99-2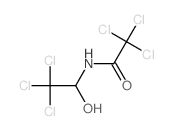 CAS#:18271-89-1
CAS#:18271-89-1 CAS#:28460-70-0
CAS#:28460-70-0![2,2,2-trichloro-N-[(2,2,2-trichloroacetyl)sulfamoyl]acetamide structure](https://image.chemsrc.com/caspic/365/26268-56-4.png) CAS#:26268-56-4
CAS#:26268-56-4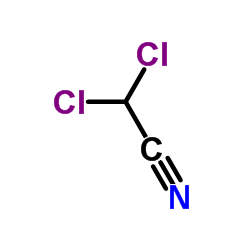 CAS#:3018-12-0
CAS#:3018-12-0 CAS#:683-72-7
CAS#:683-72-7![Thiophene,1,1,2,3,4,5-hexahydro-1-[(trichloroacetyl)imino]- (8CI,9CI) structure](https://image.chemsrc.com/caspic/119/15436-35-8.png) CAS#:15436-35-8
CAS#:15436-35-8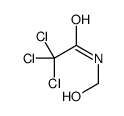 CAS#:34891-76-4
CAS#:34891-76-4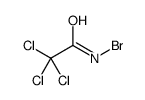 CAS#:35077-12-4
CAS#:35077-12-4
