2-Aminoethanethiol
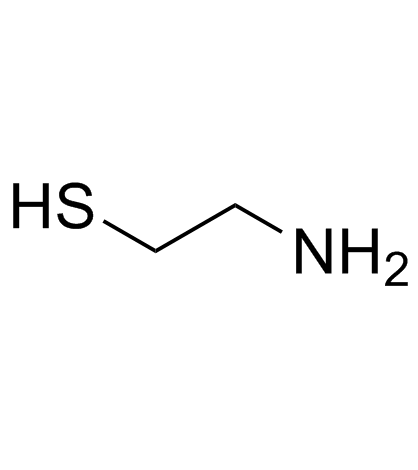
2-Aminoethanethiol structure
|
Common Name | 2-Aminoethanethiol | ||
|---|---|---|---|---|
| CAS Number | 60-23-1 | Molecular Weight | 77.149 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 133.6±23.0 °C at 760 mmHg | |
| Molecular Formula | C2H7NS | Melting Point | 95°C | |
| MSDS | USA | Flash Point | 34.6±22.6 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 2-AminoethanethiolCysteamine is an agent for the treatment of nephropathic cystinosis and an antioxidant.Target: OthersCysteamine has been shown to increase intracellular glutathione levels in cystinotic cells, thus restoring the altered redox state of the cells. Also increased rates of apoptosis in cystinotic cells, which are thought to be the result of increased caspase 3 and protein kinase Cε activity, is counteracted by Cysteamine administration. Cysteamine has antioxidant properties as a result of increasing glutathione production. Cysteamine is an excellent scavenger of OH and HOCl; it also reacts with H2O2. Cysteamine increases the production of several heat shock proteins (HSP), including the murine Hsp40. Cysteamine exerts a dose-dependent effect on the doxorubicin-induced death of cancer cells, measured in both HeLa cells and B16 cells, whereas Cysteamine treatment alone had no influence on cell survival. In addition, in a doxorubicin-resistant breast cancer cell line, the addition of Cysteamine to doxorubicin results in a dramatic increase in cell death [1]. Cysteamine (100 μM) significantly is able to increase the intracellular GSH levels and the percentage of embryos that developed to the blastocyst stage of culture matured oocytes [2]. |
| Name | cysteamine |
|---|---|
| Synonym | More Synonyms |
| Description | Cysteamine is an agent for the treatment of nephropathic cystinosis and an antioxidant.Target: OthersCysteamine has been shown to increase intracellular glutathione levels in cystinotic cells, thus restoring the altered redox state of the cells. Also increased rates of apoptosis in cystinotic cells, which are thought to be the result of increased caspase 3 and protein kinase Cε activity, is counteracted by Cysteamine administration. Cysteamine has antioxidant properties as a result of increasing glutathione production. Cysteamine is an excellent scavenger of OH and HOCl; it also reacts with H2O2. Cysteamine increases the production of several heat shock proteins (HSP), including the murine Hsp40. Cysteamine exerts a dose-dependent effect on the doxorubicin-induced death of cancer cells, measured in both HeLa cells and B16 cells, whereas Cysteamine treatment alone had no influence on cell survival. In addition, in a doxorubicin-resistant breast cancer cell line, the addition of Cysteamine to doxorubicin results in a dramatic increase in cell death [1]. Cysteamine (100 μM) significantly is able to increase the intracellular GSH levels and the percentage of embryos that developed to the blastocyst stage of culture matured oocytes [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 133.6±23.0 °C at 760 mmHg |
| Melting Point | 95°C |
| Molecular Formula | C2H7NS |
| Molecular Weight | 77.149 |
| Flash Point | 34.6±22.6 °C |
| Exact Mass | 77.029922 |
| PSA | 64.82000 |
| LogP | 0.03 |
| Vapour Pressure | 8.4±0.2 mmHg at 25°C |
| Index of Refraction | 1.486 |
| InChIKey | UFULAYFCSOUIOV-UHFFFAOYSA-N |
| SMILES | NCCS |
| Stability | Stable, but may be air-sensitive. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | 3259 |
| WGK Germany | 3 |
| RTECS | KJ0175000 |
| Precursor 9 | |
|---|---|
| DownStream 9 | |
|
Mediated Electron Transfer at Vertically Aligned Single-Walled Carbon Nanotube Electrodes During Detection of DNA Hybridization.
Nanoscale Res. Lett. 10 , 978, (2015) Vertically aligned single-walled carbon nanotube (VASWCNT) assemblies are generated on cysteamine and 2-mercaptoethanol (2-ME)-functionalized gold surfaces through amide bond formation between carboxy... |
|
|
A nanoplasmonic biosensor for label-free multiplex detection of cancer biomarkers.
Biosens. Bioelectron. 74 , 341-6, (2015) The development of highly sensitive, selective and multiplex sensors has become an important challenge for disease diagnosis. In this study, we describe a multiplex biosensor for the detection of canc... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI typ... |
| b-MEA |
| Ethanethiol, 2-amino- |
| 2-Aminoethanethiol |
| Thioethanolamine |
| β-aminoethylthiol |
| β-mercaptoethylamine |
| Mercamine |
| MEA |
| mercaptoethylamine |
| mercaptamine |
| b-Aminoethylthiol |
| Merkamin |
| b-Aminoethanethiol |
| Mercaptamin |
| 2-mercaptoethylamine |
| b-Mercaptoethylamine |
| MFCD00008196 |
| cysteamine |
| Decarboxycysteine |
| β-Aminoethanethiol |
| EINECS 200-463-0 |
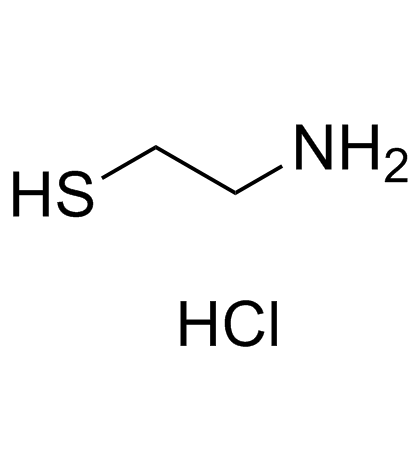 CAS#:156-57-0
CAS#:156-57-0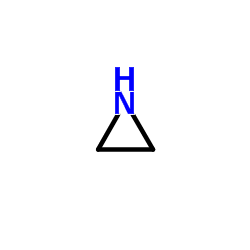 CAS#:151-56-4
CAS#:151-56-4![Ethanamine,2-[(triphenylmethyl)thio]- Structure](https://image.chemsrc.com/caspic/082/1095-85-8.png) CAS#:1095-85-8
CAS#:1095-85-8 CAS#:7783-06-4
CAS#:7783-06-4![1-thia-4-azaspiro[4.5]decane Structure](https://image.chemsrc.com/caspic/415/177-07-1.png) CAS#:177-07-1
CAS#:177-07-1 CAS#:2937-53-3
CAS#:2937-53-3 CAS#:52-90-4
CAS#:52-90-4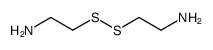 CAS#:51-85-4
CAS#:51-85-4 CAS#:70-18-8
CAS#:70-18-8 CAS#:108-02-1
CAS#:108-02-1 CAS#:4569-82-8
CAS#:4569-82-8 CAS#:31404-08-7
CAS#:31404-08-7 CAS#:1420-88-8
CAS#:1420-88-8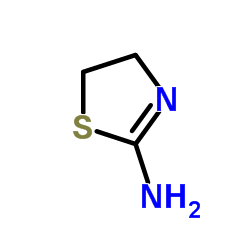 CAS#:1779-81-3
CAS#:1779-81-3 CAS#:504-78-9
CAS#:504-78-9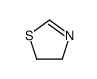 CAS#:504-79-0
CAS#:504-79-0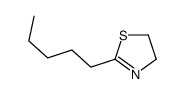 CAS#:21226-52-8
CAS#:21226-52-8
