2,4,6-Triiodophenol
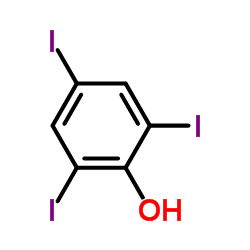
2,4,6-Triiodophenol structure
|
Common Name | 2,4,6-Triiodophenol | ||
|---|---|---|---|---|
| CAS Number | 609-23-4 | Molecular Weight | 471.801 | |
| Density | 3.1±0.1 g/cm3 | Boiling Point | 316.3±42.0 °C at 760 mmHg | |
| Molecular Formula | C6H3I3O | Melting Point | 157-159 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 145.1±27.9 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 2,4,6-Triiodophenol2,4,6-Triiodophenol is an orally active and potent leukotriene B4 (LTB4) synthesis inhibitor. 2,4,6-Triiodophenol can induce mouse blastocysts apoptosis[1][2]. |
| Name | 2,4,6-Triiodophenol |
|---|---|
| Synonym | More Synonyms |
| Description | 2,4,6-Triiodophenol is an orally active and potent leukotriene B4 (LTB4) synthesis inhibitor. 2,4,6-Triiodophenol can induce mouse blastocysts apoptosis[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | 2,4,6-Triiodophenol (5-50 μM; 0-100 h) impairs the quality of pre-implantation mouse embryos in a dose-dependent manner, inducing decline of both total and trophectoderm cell numbers[2]. 2,4,6-Triiodophenol (5 μM; 85 h) shows increasement of apoptotic cells in mouse pre-implantation embryos[2]. 2,4,6-Triiodophenol (5-50 μM; 100 h) induces oxidative stress in mouse pre-implantation embryos[2]. Apoptosis Analysis[2] Cell Line: Mouse blastocyst cells Concentration: 5 μM Incubation Time: 85 hours Result: Showed signals of activated Caspase-3/7. Immunofluorescence[2] Cell Line: Mouse blastocyst cells Concentration: 5-50 μM Incubation Time: 100 hours Result: Resulted in marked increase of oxygen species (ROS) treated with high dose of 2,4,6-Triiodophenol. |
| Density | 3.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 316.3±42.0 °C at 760 mmHg |
| Melting Point | 157-159 °C(lit.) |
| Molecular Formula | C6H3I3O |
| Molecular Weight | 471.801 |
| Flash Point | 145.1±27.9 °C |
| Exact Mass | 471.731781 |
| PSA | 20.23000 |
| LogP | 3.88 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.816 |
| InChIKey | VAPDZNUFNKUROY-UHFFFAOYSA-N |
| SMILES | Oc1c(I)cc(I)cc1I |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H312 + H332-H315-H319-H335 |
| Precautionary Statements | P261-P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | SN2800000 |
| HS Code | 2908199090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2908199090 |
|---|---|
| Summary | HS: 2908199090. derivatives of polyphenols or phenol-alcohols containing only halogen substituents and their salts. VAT:17.0%. tax rebate rate:9.0%. supervision conditions:None. MFN tariff:5.5%. general tariff:30.0% |
|
Distribution of dehalogenation activity in subseafloor sediments of the Nankai Trough subduction zone.
Philos. Trans. R. Soc. Lond., B, Biol. Sci. 368(1616) , 20120249, (2013) Halogenated organic matter buried in marine subsurface sediment may serve as a source of electron acceptors for anaerobic respiration of subseafloor microbes. Detection of a diverse array of reductive... |
|
|
Comparative developmental toxicity of new aromatic halogenated DBPs in a chlorinated saline sewage effluent to the marine polychaete Platynereis dumerilii.
Environ. Sci. Technol. 47(19) , 10868-76, (2013) Using seawater for toilet flushing may introduce high levels of bromide and iodide into a city's sewage treatment works, and result in the formation of brominated and iodinated disinfection byproducts... |
|
|
Fast speciation analysis of iodophenol compounds in river waters by capillary electrophoresis-inductively coupled plasma-mass spectrometry with off-line solid-phase microextraction.
Electrophoresis 25(12) , 1843-51, (2004) An analytical methodology for the fast separation and determination of iodophenol species in natural water samples was developed using capillary electrophoresis (CE) coupled to inductively coupled pla... |
| 2,6-Triiodophenol |
| 2,4,6-Triiodophenol |
| Phenol,4,6-triiodo |
| 2,4,6-Trijodfenol |
| triiodo-2,4,6 phenol |
| 2,3-DIHYDROXYL QUINOXALINE |
| Phenol, 2,4,6-triiodo- |
| Phenol,2,4,6-triiodo |
| EINECS 210-186-7 |
| 2,4,6-Trijodfenol [Czech] |
| 2,4,6-triIPhOH |
| MFCD00002179 |
| 2,4,6-triiodo-phenol |
 CAS#:108-95-2
CAS#:108-95-2 CAS#:98-80-6
CAS#:98-80-6 CAS#:69-72-7
CAS#:69-72-7 CAS#:7553-56-2
CAS#:7553-56-2 CAS#:554-71-2
CAS#:554-71-2 CAS#:99-96-7
CAS#:99-96-7 CAS#:139-02-6
CAS#:139-02-6 CAS#:119-30-2
CAS#:119-30-2 CAS#:618-76-8
CAS#:618-76-8 CAS#:133-91-5
CAS#:133-91-5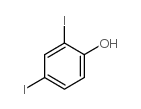 CAS#:2012-29-5
CAS#:2012-29-5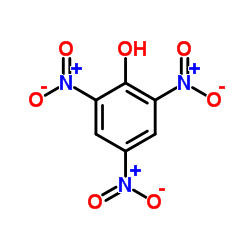 CAS#:88-89-1
CAS#:88-89-1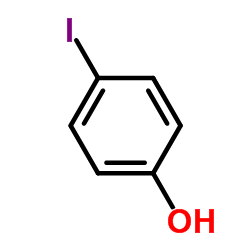 CAS#:540-38-5
CAS#:540-38-5 CAS#:118-75-2
CAS#:118-75-2 CAS#:87-86-5
CAS#:87-86-5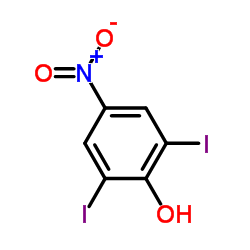 CAS#:305-85-1
CAS#:305-85-1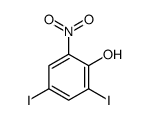 CAS#:20294-48-8
CAS#:20294-48-8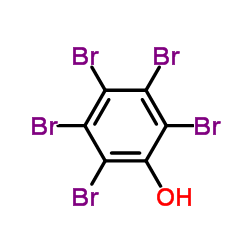 CAS#:608-71-9
CAS#:608-71-9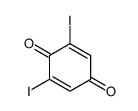 CAS#:20389-01-9
CAS#:20389-01-9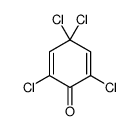 CAS#:20244-55-7
CAS#:20244-55-7