4'-Hydroxy diclofenac
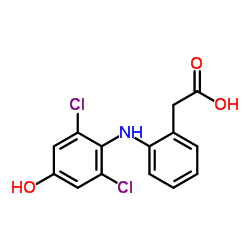
4'-Hydroxy diclofenac structure
|
Common Name | 4'-Hydroxy diclofenac | ||
|---|---|---|---|---|
| CAS Number | 64118-84-9 | Molecular Weight | 312.148 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 432.7±45.0 °C at 760 mmHg | |
| Molecular Formula | C14H11Cl2NO3 | Melting Point | 178-185ºC dec. | |
| MSDS | Chinese USA | Flash Point | 215.5±28.7 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
Use of 4'-Hydroxy diclofenac4'-Hydroxy diclofenac is an orally active metabolite of Diclofenac (HY-15036) by cytochrome P450 2C9 (CYP2C9). 4'-Hydroxy diclofenac has anti-inflammatory and analgesic properties[1][2]. |
| Name | 4'-hydroxydiclofenac |
|---|---|
| Synonym | More Synonyms |
| Description | 4'-Hydroxy diclofenac is an orally active metabolite of Diclofenac (HY-15036) by cytochrome P450 2C9 (CYP2C9). 4'-Hydroxy diclofenac has anti-inflammatory and analgesic properties[1][2]. |
|---|---|
| Related Catalog | |
| In Vivo | A single oral administration of Diclofenac to humanized mice, the unchanged drug in plasma peaks at 0.25 hour and then declines with a half-life (t1/2) of 2.4 hours. 4'-Hydroxy diclofenac also peaks at 0.25 hour and is undetectable within 24 hours. The plasma concentration of unchanged 4'-Hydroxy diclofenac peaks at 0.25 hour and declines rapidly in Humanized chimeric mice received of 4'-Hydroxy diclofenac (10 mg/kg; a single oral)[2]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 432.7±45.0 °C at 760 mmHg |
| Melting Point | 178-185ºC dec. |
| Molecular Formula | C14H11Cl2NO3 |
| Molecular Weight | 312.148 |
| Flash Point | 215.5±28.7 °C |
| Exact Mass | 311.011597 |
| PSA | 69.56000 |
| LogP | 4.56 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.690 |
| InChIKey | KGVXVPRLBMWZLG-UHFFFAOYSA-N |
| SMILES | O=C(O)Cc1ccccc1Nc1c(Cl)cc(O)cc1Cl |
| Storage condition | −20°C |
| Water Solubility | Soluble in DMSO, ethanol or methanol |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H302 + H312 + H332-H319 |
| Precautionary Statements | P210-P261-P302 + P352 + P312-P304 + P340 + P312-P337 + P313-P403 + P235 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic;N: Dangerous for the environment; |
| Risk Phrases | R25;R37/38;R41;R50/53 |
| Safety Phrases | S26-S39-S45-S60-S61 |
| RIDADR | UN 2811 |
| WGK Germany | 3 |
| RTECS | AG6542800 |
| HS Code | 2922509090 |
| Precursor 9 | |
|---|---|
| DownStream 1 | |
| HS Code | 2922509090 |
|---|---|
| Summary | 2922509090. other amino-alcohol-phenols, amino-acid-phenols and other amino-compounds with oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
High warfarin sensitivity in carriers of CYP2C9*35 is determined by the impaired interaction with P450 oxidoreductase.
Pharmacogenomics J. 14(4) , 343-9, (2014) Cytochrome P450 2C9 (CYP2C9) metabolizes many clinically important drugs including warfarin and diclofenac. We have recently reported a new allelic variant, CYP2C9*35, found in a warfarin hypersensiti... |
|
|
Inhibition of human cytochrome P450 enzymes by licochalcone A, a naturally occurring constituent of licorice.
Toxicol. In Vitro 29 , 1569-76, (2015) Licochalcone A (LCA) is a major bioactive compound in traditional Chinese herbal liquorice that possesses multiple pharmacological activities. However, the effects of the potential herb-drug interacti... |
|
|
Structural basis for the 4'-hydroxylation of diclofenac by a microbial cytochrome P450 monooxygenase.
Appl. Microbiol. Biotechnol. 99(7) , 3081-91, (2015) Diclofenac is a nonsteroidal anti-inflammatory drug. It undergoes hydroxylation by mammalian cytochrome P450 enzymes at 4'- and/or 5'-positions. A bacterial P450 enzyme, CYP105D7 from Streptomyces ave... |
| 4’-Hydroxy Diclofenac |
| 4'-hydroxydiclofenac |
| 4'-Hydroxy Diclofenac |
| Benzeneacetic acid, 2-[(2,6-dichloro-4-hydroxyphenyl)amino]- |
| {2-[(2,6-Dichloro-4-hydroxyphenyl)amino]phenyl}acetic acid |
 CAS#:15307-86-5
CAS#:15307-86-5![2-[2-(2',6'-DICHLORO-4'-HYDROXPHENYLAMINO)PHENYL]-N,N-DIMETHYLACETAMIDE Structure](https://image.chemsrc.com/caspic/377/698357-45-8.png) CAS#:698357-45-8
CAS#:698357-45-8 CAS#:73328-71-9
CAS#:73328-71-9 CAS#:15307-79-6
CAS#:15307-79-6 CAS#:637-62-7
CAS#:637-62-7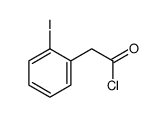 CAS#:62300-07-6
CAS#:62300-07-6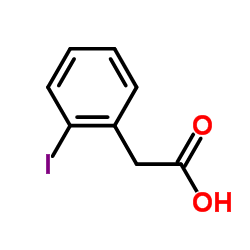 CAS#:18698-96-9
CAS#:18698-96-9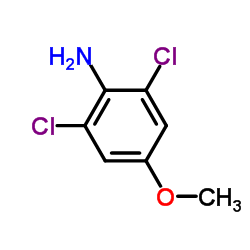 CAS#:6480-66-6
CAS#:6480-66-6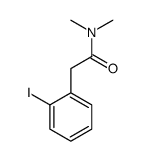 CAS#:75117-26-9
CAS#:75117-26-9