Altretamine
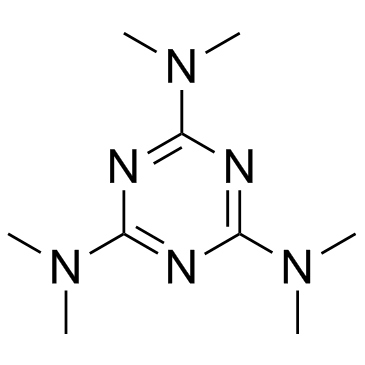
Altretamine structure
|
Common Name | Altretamine | ||
|---|---|---|---|---|
| CAS Number | 645-05-6 | Molecular Weight | 210.279 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 339.4±25.0 °C at 760 mmHg | |
| Molecular Formula | C9H18N6 | Melting Point | 171-175 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 159.1±23.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of AltretamineAltretamine is an alkylating antineoplastic agent. |
| Name | hexamethylmelamine |
|---|---|
| Synonym | More Synonyms |
| Description | Altretamine is an alkylating antineoplastic agent. |
|---|---|
| Related Catalog | |
| In Vitro | Altretamine is an antineoplastic agent[1]. |
| In Vivo | Altretamine (100, 133 mg/kg, ip.) in combination with Irofulven, increases the antitumor effect in mice bearing MV522 cells[1]. |
| Animal Admin | Mice[1] Balb/c nu/nu 4 week old female mice weighing 18-22 g, receive s.c. injections of 8-10 million MV522 cells. Altretamine is administered i.p. three times a week for 3 weeks, starting on day 10 after tumor implantation. Tumor size is measured in two perpendicular diameters and tumor weight (TW) estimated according to the formula: w = [(width)2 × length/2]. Altretamine is prepared as stock solutions of 1-10 mg/mL in 40% DMSO/normal saline and diluted with 10% DMSO/normal saline as required[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 339.4±25.0 °C at 760 mmHg |
| Melting Point | 171-175 °C(lit.) |
| Molecular Formula | C9H18N6 |
| Molecular Weight | 210.279 |
| Flash Point | 159.1±23.2 °C |
| Exact Mass | 210.159302 |
| PSA | 48.39000 |
| LogP | 2.42 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.610 |
| InChIKey | UUVWYPNAQBNQJQ-UHFFFAOYSA-N |
| SMILES | CN(C)c1nc(N(C)C)nc(N(C)C)n1 |
| Storage condition | Refrigerator |
| Water Solubility | Insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | OS1050000 |
| HS Code | 2933699090 |
| HS Code | 2933699090 |
|---|---|
| Summary | 2933699090 other compounds containing an unfused triazine ring (whether or not hydrogenated) in the structure。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:20.0% |
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
|
|
Quantitative structure-activity relationship and complex network approach to monoamine oxidase A and B inhibitors.
J. Med. Chem. 51 , 6740-51, (2008) The work provides a new model for the prediction of the MAO-A and -B inhibitor activity by the use of combined complex networks and QSAR methodologies. On the basis of the obtained model, we prepared ... |
|
|
FDA-approved drug labeling for the study of drug-induced liver injury.
Drug Discov. Today 16 , 697-703, (2011) Drug-induced liver injury (DILI) is a leading cause of drugs failing during clinical trials and being withdrawn from the market. Comparative analysis of drugs based on their DILI potential is an effec... |
| HTM |
| 1,3,5-Triazine-2,4,6-triamine, N,N,N,N,N,N-hexamethyl- |
| Hexastat |
| 2,4,6-tris<dimethylamino>-1,3,5-triazine |
| HMM |
| N2,N2,N4,N4,N6,N6-hexamethyl-1,3,5-triazine-2,4,6-triamine |
| Hexalen |
| EINECS 211-428-4 |
| N,N,N’,N’,N″,N″-hexamethyl-1,3,5-triazine-2,4,6-triamine |
| N,N,N',N',N'',N''-Hexamethyl-1,3,5-triazine-2,4,6-triamine |
| hexamethylmelamine |
| Altretamine |
| Hexylen |
| MFCD00549245 |
| N,N,N,N,N,N-Hexamethyl-1,3,5-triazine-2,4,6-triamine |
| NC 195 |
| hemel |
 CAS#:1467-79-4
CAS#:1467-79-4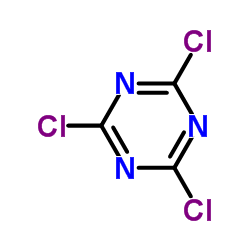 CAS#:108-77-0
CAS#:108-77-0 CAS#:124-40-3
CAS#:124-40-3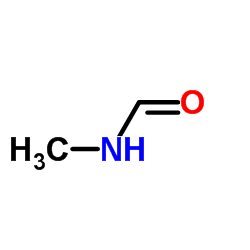 CAS#:123-39-7
CAS#:123-39-7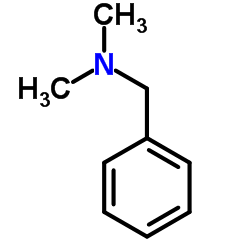 CAS#:103-83-3
CAS#:103-83-3 CAS#:51-80-9
CAS#:51-80-9 CAS#:506-59-2
CAS#:506-59-2 CAS#:108-80-5
CAS#:108-80-5
